2012F5HKDSE_F5_Expt_..
advertisement

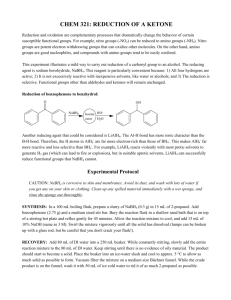
Reduction of Ketone by NaBH4 Reduction of a ketone (酮的還原反應) Name __________ Class ___ ( ) Introduction Aldehydes and ketones can undergo reduction with metal hydrides, e.g. LiAlH 4 or NaBH4. The discovery of the reducing powers of NaBH4 occurred accidentally by Prof. H.C. Brown. NaBH4 is relative mild, alcohols can be chosen as solvents. LiAlH4 is sensitive to air and moisture, dry ether is chosen as solvent. Usually less reactive NaBH4 is commonly used in the reduction of carbonyl compounds. Chemicals Benzophenone solids, NaBH4 solids, propan-2-ol, 10% NaOH(aq), Dichloromethane, 2,4-dinitrophenylhydrazine solution, concentrated HCl(aq),0.1 M ZnCl2(aq), hexane Apparatus Quick-fit apparatus, separating funnel, 50 cm3 conical flask Precautions: NaBH4 solids react with water to give hydrogen gas which is flammable, propan-2-ol is flammable, dichloromethane is harmful. Procedure : Add 1.0 g of benzophenone solids and 0.2 g of NaBH4 solids into a 50 cm3 dry pear-shaped flask respectively. Pour 10 cm3 propan-2-ol in it. A few pieces of pumice stone is added to the mixture. Heat the mixture under reflux for five minutes. Cool the mixture to room temperature, add 10 cm3 of 10% NaOH(aq) solution into the pear-shaped flask. Transfer the total content into a separating funnel, rinse the pear-shaped flask with 10 cm3 of water. Pour the rinsing water into the separating funnel. Extract the aqueous layer with two portions of 8 cm3 of dichloromethane. Dry the combined extracts with anhydrous sodium sulphate solid . Transfer the solution into a 50 cm3 round-bottomed flask. Remove dichloromethane and the remaining propan-2-ol by simple distillation. A wax-like solid will be formed as the crude product. Dissolve the crude product with a minimum amount of hot hexane in the round-bottomed flask and pour the hot solution in a conical flask. Divide the hot hexane solution into two portions. One portion is cooled slowly at room temperature. The other portion is placed into an ice-water bath for cooling rapidly. (Recrystallisation 重結晶) Record the mass of product obtained. Measure the melting point of the product with a B-tube. Record the infra-red (IR) spectrum of the product by FT-IR spectrometer. Dissolve a small amount of product in hexane and dissolve a small amount of benzophenone in hexane respectively. Carry out the following chemical tests. Chemical test (1) : add the prepared solutions to an acidified K2Cr2O7(aq) solution. Chemical test (2) : add two drops of ZnCl2(aq) and two drops of conc. HCl(aq) to the solutions prepared. Chemical test (3) : add two drops of 2,4-dinitrophenylhydrazine solution to the solutions prepared. 1 Reduction of Ketone by NaBH4 Reduction of a ketone (酮的還原反應) Name __________ Class ___ ( ) Mark _________ Describe the appearance of the recrystallised solid (by cooling rapidly) = ____________________ Describe the appearance of the recrystallised solid (by cooling slowly) = ____________________ Melting point of the product = ____________________ Mass of benzophenone used / g = ___________________ Mass of product obtained / g = ___________________ Calculate the percentage yield of the reaction. Observation of the chemical tests performed Reagent Benzophenone Product acidified K2Cr2O7(aq) solution ZnCl2(aq) and conc. HCl(aq) (Lucas reagent) 2,4-dinitrophenylhydrazine Discussion: 1. Write the structures of benzophenone and the organic product respectively. 2. Give the systematic names of the organic product respectively. 2 Reduction of Ketone by NaBH4 3. 4. When the mixture of benzophenone and NaBH4 is heated under reflux, (i) explain why pumice stone (浮石) is added to the mixture? (ii) draw a labelled diagram for the assembly of experimental set-up. Compare the infra-red spectrum of benzophenone and that of the organic product, what information about the conversion of the functional group would be obtained ? Explain your answer. 5. Based on results of the chemical tests, what information about the conversion of the functional group would be obtained ? Explain your answer. 3 Reduction of Ketone by NaBH4 Marking Scheme 1. 2. 3. 4. 5. 6. 7. 8. Appearance of recrystallised solid Melting point = 58 – 68 oC Calculation of percentage yield IR spectrum of the product obtained Observation of chemical tests The function of pumice stones is to prevent the reaction mixture from bumping. IR of benzophenone : absorption peak at 1700 cm1, no broad absorption peak at 3300 3500 cm1 (in the presence of C=O group) (without OH group) 1 1 1 1 1 1 1 IR of product : no absorption peak at 1700 cm1, a broad absorption peak at 3300 3500 cm1 (without C=O group) (in the presence of OH group) 1 The product has a OH group Acidified K2Cr2O7(aq) test : orange solution turns green HCl(aq) and ZnCl2(aq) test : white solid forms 2,4-dinitrophenylhydrazine test : negative result 1 1 The C=O group is reduced to COH group by NaBH4. (1) 4