handout aldol condensation
advertisement

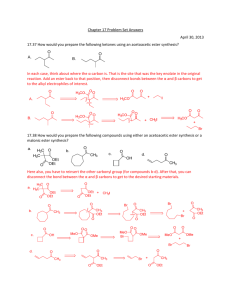
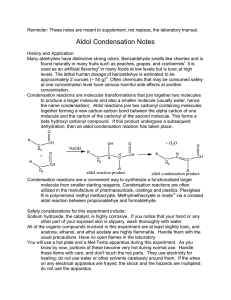
Name Lab Section GTA Station # 6. Aldol Reaction Pre-lab questions Complete the following questions and submit before beginning your experiment. 1. In order to produce dibenzalacetone and not benzalacetone, what should the limiting reagent in this reaction be? 2. Why are steps 4, 5, and 6 needed in the procedure of the experiment? Pre-lab Notebook preparation Prepare the following in your lab notebook before beginning the experiment. 1. Start a new page by entering the title of this experiment. 2. Start a section with the subtitle “Procedure” and provide an outline of the experimental procedure to be carried out. Aldol Reaction 1 6. Aldol reaction Jones: Condensation Reactions, Aldol reaction, Chapter 17, Section 3, pgs 840-850. McMurry: Condensation Reactions, Aldol reaction, Chapter 11, Section 8, pgs 362-364. This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). Background Aldol Reaction. In the experiment, you will perform a base-catalyzed, condensation reaction using benzaldehyde and acetone (see Figure 1). A condensation reaction is one which condenses two or more molecules to make one single compound. O C O H + 2 Benzaldehyde bp 178 o C M.W. 106.13 density 1.04 g/mL H 3C CH3 Acetone (2-propanone) bp 56 oC M.W. 58.08 density 0.790 g/mL NaOH M.W. 40.01 H C O C H C H H C Dibenzalacetone (1,5-Diphenyl-1,4pentadien-3-one), mp 42 oC Figure 1. The overall reaction. First let's define some different types of compounds. An aldehyde is a molecule with an R-HC=O structure. There is a carbonyl group (C=O). The carbon of the carbonyl group is flanked by a hydrogen (H) and an R group (R does not equal H). A ketone is a molecule with an R2C=O. The R groups do not have to be the same. In this reaction, two molecules of benzaldehyde (aldehyde) are condensed with one molecule of acetone (ketone). Acetone acts as a nucleophile which adds to the carbonyl carbon of benzaldehyde). The first step in the reaction is to form Aldol Reaction 2 the nucleophile, which is an enolate ion. This is a deprotonation step where a hydroxide ion pulls off a proton from the alpha carbon (carbon adjacent to the carbonyl carbon) to yield a resonance-stabilized enolate. The nucleophile adds to the carbonyl carbon of benzaldehyde in step 2. The resulting alkoxide ion is protonated in step 3 to form the "true" Aldol product which has both alcohol (OH) and carbonyl (C=O) functionalities. With heating, this product eliminates water (dehydration) to form an-unsaturated ketone. This happens first by a deprotonation step (step 4) with sodium hydroxide to form a resonance-stabilized carbanion. Then in step 5, a hydroxide ion is eliminated to form the unsaturated ketone called benzalacetone. The entire reaction sequence is repeated to condense another molecule of benzaldehyde to the second alpha carbon of acetone and form dibenzalacetone. H 3C O O O CH2 H H 3C CH2 H 3C CH2 + H 2O resonance forms of enolate OH step 1: formation of the enolate H OH O C O H H 3C CH2 step 2: addition of enolate to PhCOH OH C H O CH H CH3 O C H O H 2C CH3 step 3: protonation of alkoxide OH C H O HC CH3 + H 2O + H 2O step 4: deprotonation of alpha carbon OH H C O C H CH3 step 5: elimination of hydroxide Figure 2. General reaction mechanism for the condensation of one molecule of benzaldehyde with one molecule of acetone. Aldol Reaction 3 Experiment 1. Prepare an ice water bath for the recrystallization step (step 8). 2. Transfer your different solutions (7 mL of 5 M sodium hydroxide-NaOH, 10 mL of ethanol-CH3CH2OH, 0.5 mL of acetone-CH3COCH3, and 1.5 mL of benzaldehyde-PhCHO) into your reaction tube and cap the tube. Make sure to record all amounts of starting materials. 3. Shake the tube a few times every minute for a total of 30 minutes. 4. After that time period, remove the liquid from the crystals using a pipette. 5. Add 10 mL of water and shake the tube. 6. Remove the water with a pipette and repeat steps 4 and 5 two more times. 7. Collect the crystals by vacuum filtration. 8. Recrystallize the resulting crystals using an ethanol/water solvent pair. 9. Cool the solution to room temperature. Then put the reaction tube in an ice water bath. 10. Vacuum filter and wash the crystals with a cold solution of ethanol/water (70:30). 11. Dry the crystals, weigh the crystals, take a melting point and report the percent yield. Tips: * Before you start the experiment, start the water bath and put ethanol on ice to save time. * If no crystals form, scratch the side of the tube with a stir rod. For your lab reports, report the % yield and the melting point of your product. Aldol Reaction 4 Name Lab Section GTA Station # 6. Aldol Reaction Post-lab report Fill out the appropriate sections below. Initial volume of benzaldehyde (Vbi in mL) Initial weight of benzaldehyde (Wbi in mL) Initial amount of benzaldehyde (Mbi in moles) Initial volume of acetone (Vai in mL) Initial weight of acetone (Wai in mL) Initial amount of acetone (Mai in moles) Identification of limiting reagent Theoretical amount of dibenzalacetone based on limiting reagent (T in moles) Final Weight of dibenzalacetone (Wf in grams) Final amount of dibenzalacetone (Mf in moles) % yield melting point (°C) Aldol Reaction 5 Questions: 1. Draw the product(s) for the following reactions: O C a) + O C b) O H H 3C C(CH3) 3 NaOH Ethanol CH3 NaOH Ethanol O H + H 3C Aldol Reaction 6