NaBH4 Reduction of Ketone to Alcohol: Lab Experiment
advertisement
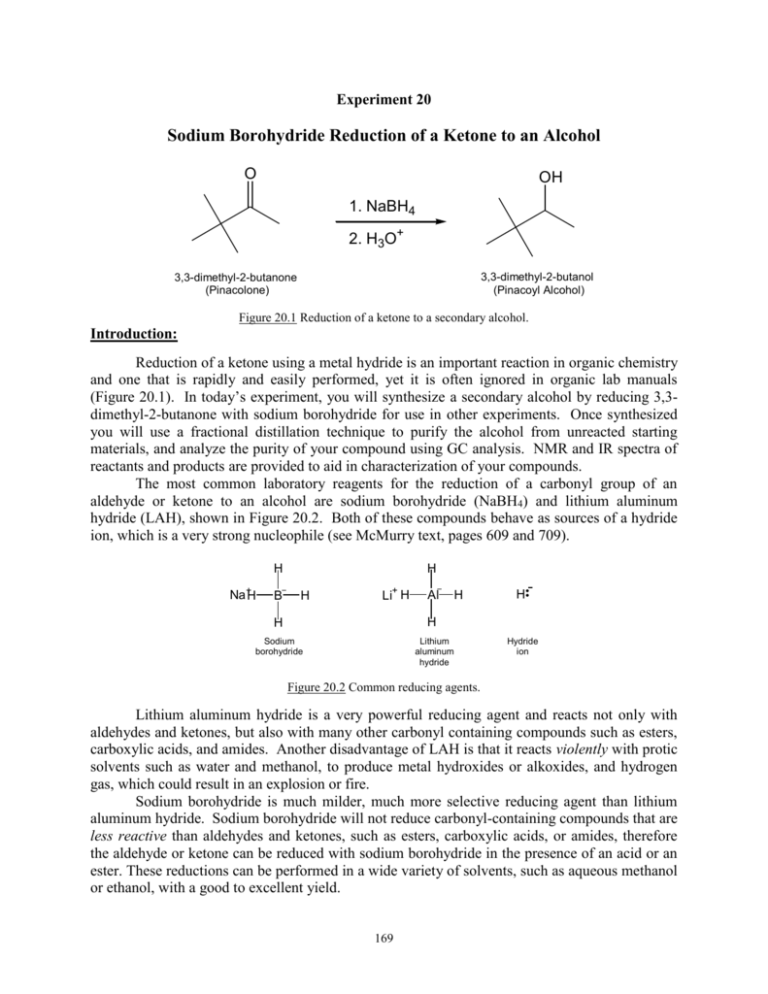
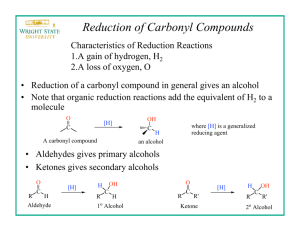
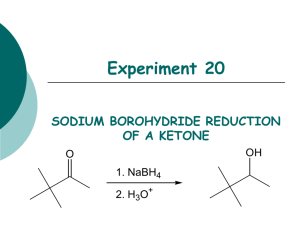
Experiment 20 Sodium Borohydride Reduction of a Ketone to an Alcohol O OH 1. NaBH4 2. H3O+ 3,3-dimethyl-2-butanol (Pinacoyl Alcohol) 3,3-dimethyl-2-butanone (Pinacolone) Figure 20.1 Reduction of a ketone to a secondary alcohol. Introduction: Reduction of a ketone using a metal hydride is an important reaction in organic chemistry and one that is rapidly and easily performed, yet it is often ignored in organic lab manuals (Figure 20.1). In today’s experiment, you will synthesize a secondary alcohol by reducing 3,3dimethyl-2-butanone with sodium borohydride for use in other experiments. Once synthesized you will use a fractional distillation technique to purify the alcohol from unreacted starting materials, and analyze the purity of your compound using GC analysis. NMR and IR spectra of reactants and products are provided to aid in characterization of your compounds. The most common laboratory reagents for the reduction of a carbonyl group of an aldehyde or ketone to an alcohol are sodium borohydride (NaBH4) and lithium aluminum hydride (LAH), shown in Figure 20.2. Both of these compounds behave as sources of a hydride ion, which is a very strong nucleophile (see McMurry text, pages 609 and 709). H H Na+H B H Li+ H Al H H H H Sodium borohydride Lithium aluminum hydride Hydride ion Figure 20.2 Common reducing agents. Lithium aluminum hydride is a very powerful reducing agent and reacts not only with aldehydes and ketones, but also with many other carbonyl containing compounds such as esters, carboxylic acids, and amides. Another disadvantage of LAH is that it reacts violently with protic solvents such as water and methanol, to produce metal hydroxides or alkoxides, and hydrogen gas, which could result in an explosion or fire. Sodium borohydride is much milder, much more selective reducing agent than lithium aluminum hydride. Sodium borohydride will not reduce carbonyl-containing compounds that are less reactive than aldehydes and ketones, such as esters, carboxylic acids, or amides, therefore the aldehyde or ketone can be reduced with sodium borohydride in the presence of an acid or an ester. These reductions can be performed in a wide variety of solvents, such as aqueous methanol or ethanol, with a good to excellent yield. 169 The key step in the metal hydride reduction of an aldehyde or ketone is transfer of a hydride ion from the boron atom of the reducing agent to the electropositive carbon of the carbonyl group to form a tetrahedral intermediate (Figure 20.3). One mole of NaBH4 can react with 4 carbonyls to give a tetraalkoxyborate. After the intermediate is hydrolyzed with water, the tetraalkoxyborate is converted to the alcohol and boric acid salts. Thus, one mole of the reducing agent reduces four moles of the carbonyl compound. O 4 H 3C H 3C O C C CH3 CH3 H (from NaBH4) H 3C H 3C H3O+ C C CH3 H CH3 OH 4 H 3C C C H 3C CH H 3 CH3 + B(OH)3 Figure 20.3 Mechanism for the sodium borohydride reduction of a ketone. IR Spectroscopy In the IR spectra of alcohols, both the position of the OH stretch absorption and its intensity depend on the extent of hydrogen bonding between alcohol molecules. Normally, this hydrogen bonding is extensive, and the OH stretch appears as a broad peak at ~3200-3400 cm-1. The C-O stretch absorption appears in the range of 1000-1300 cm-1. Also of importance are the sp3 hybridized C-H stretch absorptions observed around 2850-3000 cm-1. Ketones show a characteristic strong IR absorption between 1705-1725 cm-1 associated with the C=O stretching absorption. Absorptions due to the sp3 hybridized C-H stretches are also present. Use the provided IR spectra (Figure 20.4 and 20.5) to characterize the ketone and the alcohol. NMR Spectroscopy The NMR spectra of the reactant and products are shown in Figure 20.4 and 20.5. The chemical shift of a hydroxyl hydrogen in proton NMR varies depending on the purity of the sample, the temperature, and the sample solvent. It typically appears in the range of 2.0-6.0 ppm, depending on the experimental conditions. Hydrogens on the carbon bearing the hydroxyl group are deshielded by the oxygen atom, and typically appear in the range of 3.5-4.5 ppm. Hydrogens attached to carbons to the carbonyl typically resonate between 2-3 ppm. One distinguishing characteristic of a methyl ketone is the presence of a large, sharp three-proton singlet around 2.1 ppm. Objectives: In this lab you will synthesize 3,3-dimethyl-2-butanol by a NaBH4 reduction of a ketone. This product will be purified using fractional distillation. The identity and purity of the product will be determined using GC analysis. Finally, you will use IR and NMR spectroscopy to distinguish between reactants and products. 170 O 1.1 9H, singlet 4 2 3 1 4 4 2.1 2.1 3H, singlet 3H, singlet 27 CHCl3 214 (NMR solvent) Figure 20.4 1H, 13C, and IR spectra of 3,3-dimethyl-2-butanone. 171 45 24 0.9 9H, singlet OH 4 2 3 1 4 1.1 3H, doublet 4 3.4 1H, quartet 2.2 1H, singlet 25 18 75 35 Figure 20.4 IR spectrum of 3,3-dimethyl-2-butanol. 1098 3400 2963 Figure 20.5 1H, 13C, and IR spectra of 3,3-dimethyl-2-butanol. 172 Experimental: Synthesis: Mix 2.0 mL of 3,3-dimethyl-2-butanone (pinacolone) and 10 mL of methanol in a 100 mL beaker. Place beaker in an ice water bath prepared in a 250 mL beaker. Add 0.60 g of NaBH4 slowly while stirring with glass rod. Allow reaction to proceed for 5 minutes. Remove beaker from ice bath and allow reaction to continue for an additional 10 minutes. SLOWLY add 2.0 mL of 6M HCl dropwise to quench the excess NaBH4. A white precipitate of boric acid should form (HCl will react with residual NaBH4 to produce H2 gas. It is important to add HCl a few drops at a time to prevent bubbling over!) Set up a suction filtration apparatus. Seat the filter paper with methanol. Slowly pour the solution into the center of the Buchner funnel to remove the solid boric acid. If a significant amount of solid appears in the liquid filtrate, filter again. If not, proceed to the next step. Using a disposable pipette, transfer the liquid filtrate to a clean 50 mL round bottom flask containing 2-3 boiling chips. Purification and Product Isolation: Prepare a fractional distillation apparatus (Appendix A). Set the VR @ 50, and collect distillate that boils below 70oC into a small flask. This distillate is mainly methanol. Switch receiving flasks to a preweighed 25 mL round bottom flask. Collect distillate that boils between 70-85oC into this flask. This fraction should contain mainly the desired product. Be sure to record the distillation range. DO NOT DISTILL TO DRYNESS! After cooling, obtain a final product weight and calculate a final % yield. Complete Table 20.1 on the final lab report. Product Analysis: GC Analysis: Prepare and submit a sample of your product for chromatographic analysis by placing 5 drops of your sample in an autoanalyzer vial and 1 mL of GC solvent (methanol). Using chromatographic results propose an identity and determine the degree of purity of your product. Complete Table 20.5 on the final lab report. IR Analysis: Using the provided, identify all characteristic absorptions of reactants and products. Complete Table 20.3 on the final lab report. NMR Analysis: Using the provided spectra, identify and tabulate all characteristic resonances of reactants and products. Complete Table 20.4 on the final lab report. WASTE MANAGEMENT Place the solid boric acid waste from the filtration into the container labeled “SOLID WASTE” located in the waste hood. Place all liquid waste into the container labeled “LIQUID WASTE”. 173 Final Lab Report Due Date_______ Names______________________________ ______________________________ Exp. 20: Sodium Borohydride Reduction of a Ketone EXPERIMENTAL RESULTS (Tables in INK only!) Table 20.1 Experimental Results Table 20.2 Green chemistry results Theoretical yield (g) Actual yield (g) Percent yield Distillation Range (oC) Product Appearance Atom Economy (%) Experimental Atom Economy (%) “Eproduct” Cost per synthesis ($) Cost per gram ($/g) Table 20.3 IR Analysis Base Values 3,3-dimethyl-2butanone Frequency (cm-1) Frequency (cm-1) 3200-3400 2850-3000 1000-1300 1700-1725 Functional Group OH stretch sp3 CH stretch C-O stretch C=O stretch 3,3-dimethyl-2butanol Frequency (cm-1) Table 20.4 NMR Analysis 3,3-dimethyl-2-butanone 3,3-dimethyl-2-butanol C1 O OH H1 C2 2 3 H1 C1 2 C2 3 1 1 4 C3 4 H4 OH 4 4 4 H2 C3 4 C4 C4 H4 Table 20.5 GC Analysis Compound GC Retention times (min) Standards Sample methanol 3,3-dimethyl-2-butanone 3,3-dimethyl-2-butanol 174 Area percent Adjusted Area Percent DISCUSSION/CONCLUSIONS (In the space provided, briefly answer the following questions. Use numerical values to support conclusions where applicable.) O NaBH4 Sodium borohydride Acros # 20005 Cost: $112.20/ 100g OH CH3OH methanol Acros # 42395 Cost: $27.00/ 500mL 3,3-dimethyl-2-butanone Acros # 13125 Cost: $21.20/ 100mL 6M HCl hydrochloric acid Acros # 42379 Cost: $27.80/ 500mL 3,3-dimethyl-2-butanol Acros # 15968 Cost: $13.10/ 10g 1. Based on GC analysis, did your sample contain pure product? Explain using actual adjusted area percent values. 2. What type of absorption band would be present in the IR spectrum of the product if unreacted 3,3-dimethyl2-butanone was present? What is the typical frequency for this type of absorption? What is the actual frequency of the absorption in the provided spectrum? 3. What is one signal in the 1H NMR spectrum that indicates that the reduction took place? What are the typical chemical shifts that these types of protons may appear? What is the actual chemical shift of this proton signal? 4. Draw the product, and a complete mechanism for its formation, for the following reaction: O H CH3 Na H3C C CH2 CHCH2CH3 H B H H **Attach sample chromatogram!** 175 176