BOROHYDRIDE
advertisement

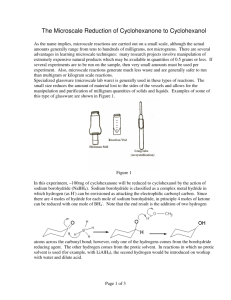
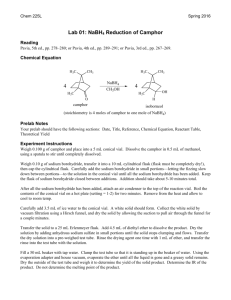
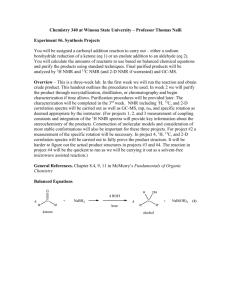
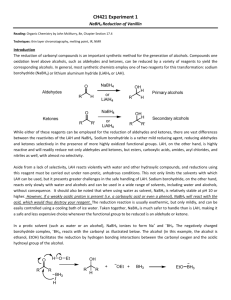
BOROHYDRIDE RECUCTION OF 9-FLUORENONE TO 9-FLUORENOL (Adapted from Missouri science and technology organic chemistry lab) “In this week’s experiment you will be utilizing a metal hydride as a reducing agent. Metal hydrides can be quite reactive, and therefore difficult, or even dangerous, to handle. Of the many hydrides explored by H. C. Brown (Nobel Prize, 1979) and co- workers at Purdue, sodium borohydride, NaBH4, (discovered in 1941), is a mild and selective reducing agent. It is safe to use in the undergraduate organic laboratory. It will be provided to you as a basic ethanolic solution (100 µL of this solution provides approximately 4.0 mg of NaBH4). The ketone to be reduced is 9-fluorenone and the resultant secondary alcohol is 9fluorenol. You will perform the reduction on about 50 mg of the ketone. The progress of the reduction is monitored by TLC (Eastman Kodak Fluorescent silica gel sheets ~ 2.5 cm x 10 cm, developed with the provided 30% acetone in hexane solution, and visualized by UV light). Weigh about 50 mg of 9-fluorenone to ± 0.001 gm in a tared 5-mL conical vial equipped with an air condenser and magnetic spin vane. Introduce about 1 mL of ethanol as solvent and stir to bring about dissolution. The NaBH4 solution is then introduced dropwise (~0.3 ml or 8 drops). After about 10 minutes, a small drop (using a capillary) is placed on the TLC sheet which has already been spotted with solutions of pure ketone and pure alcohol. (Check first semester notes and MTOL for the proper procedure for TLC analysis). If the TLC analysis reveals the presence of unreacted ketone, check with the TA to determine if more hydride reagent is needed. The color of the solution will disappear when all of the ketone is reduced. The product alcohol is isolated by first adding ~ 1.5 mL of cold 1M HCl to neutralize the alkaline reduction medium. The acid should be added dropwise as H2 gas may also be produced by the decomposition of any excess NaBH4. Check with blue litmus or pH paper to verify that excess acid is present. Separation of the alcohol as a fluffy precipitate may occur at this stage. Now you will extract the product alcohol by shaking the reaction mixture successively with one 1.0 mL and successively, two 0.5 mL portions of methylene chloride, CH2Cl2. Methylene chloride is immiscible with and more dense than water. If only a single layer is seen, add ~1 ml of saturated sodium chloride solution to help separate the layers. After each addition and shaking, the layers are allowed to separate and the lower layer of methylene chloride containing the extracted 9-fluorenol is removed with a Pasteur pipet into a dry vial. Use a small beaker or Al block to prevent the vial from tipping. If your combined mixture volume will exceed 5 ml, use a centrifuge tube to perform the extraction. The combined methylene chloride extracts are then passed through a second Pasteur pipet fitted with a cotton plug and filled with about 500 mg of anhydrous sodium sulfate into a 50 ml dry tared beaker. This operation will both dry and filter the extract. Rinse the pipet column afterwards with an additional 0.5 mL portion of methylene chloride. The combined dried eluate will be about 2 mL. The methylene chloride is then removed by gentle evaporation using a hair dryer under your mini hood. Weigh the beaker until it reaches constant weight and calculate the yield based on the mass of starting ketone. Determine the MP and hand in the product in a properly labeled vial. Procedure Summary 1. Reduce with NaBH4 solution 2. 3. 4. 5. 6. 7. Check progress by TLC (see details below) When complete, neutralize excess NaBH4 with 1M HCl Extract mixture with CH2Cl2 Dry combined CH2Cl2 extracts with Na2SO4 Remove CH2Cl2 Weigh & find MP of product TLC 1. 2. 3. 4. 5. 6. Prepare TLC tank using 30% acetone in hexane, allow vapor space to saturate. Avoid edges of plate, handle only by edges, draw pencil line ~1/2” from the bottom of the plate. Spot with reference ketone, alcohol and reaction mix, using a capillary. Develop, mark solvent front, view under UV, determine Rf’s When ketone spot is absent, reaction is complete. Reaction mix should also be colorless. Attach labeled TLC plates to yellow lab book pages with 2” clear tape Pre-Lab Questions Compare and contrast the (a) reductive abilities of LiAlH4 with those of NaBH4, and (b) compatibility of the two reducing agents with protic solvents (water, alcohols, etc.) (see Solomon’s organic text) Calculate the theoretical amount of NaBH4 needed to convert 100 mg quantities of benzil to benzoin, and benzil to hydrobenzoin. 5. Draw the structural formulas for the organic reactants and products in this week’s experiment. Which compound should move the farthest in TLC? Explain.”[15] 1. 2. 3. 4. Personal Protective Equipment Required Dusk Mask No Ear Protection No Eye Protection Yes, safety Goggles Footwear Yes, closed toed shows Gloves No Required Clothing Yes, provided lab coat Safety Consideration Chemical Reactions, When adding the HCL dropwise to the NaBH4 + 9-flourenone mixture, this should be done in a vent hood as H2 gas may be produced if there is any excess NaBH4. MSDS/SDS Yes 9-Flourenone 1. 2. 3. 4. 5. Sodium Borohydride Hydro Chloric Acid Methylene Chloride Anhydrous Sodium Sulfate 9-Flourenone - http://pubchem.ncbi.nlm.nih.gov/compound/9-Fluorenone#section=Chemical-and-Physical-Properties Sodium Borohydride -http://pubchem.ncbi.nlm.nih.gov/compound/86616055#section=Synonyms Hydro Chloric Acid - http://hazard.com/msds/f2/cld/cldfm.html Methylene Chloride - https://fscimage.fishersci.com/msds/89926.htm Anhydrous Sodium Sulfate - https://fscimage.fishersci.com/msds/21630.htm Laboratory Safety Equipment Location and Awareness Please fill in, by hand, the blank map. Be sure to include, clearly, the locations of fire extinguishers, telephones, eye wash fountains, safety shower, fume hoods, flammable storage cabinets, first-aid kit, chemical spill kit and etc. Blank Laboratory Map Laboratory Emergency Egress Emergency egress map shown below. The muster location is outside the building on the lawn nearest the egress exit. Follow current university practices regarding emergency exit (e.g., close doors but do not lock them) Butler-Carlton RM 109 Egress Map http://designconstruction.mst.edu/floorplan/ [15]