jssc4110-sup-0001-SupMat
advertisement
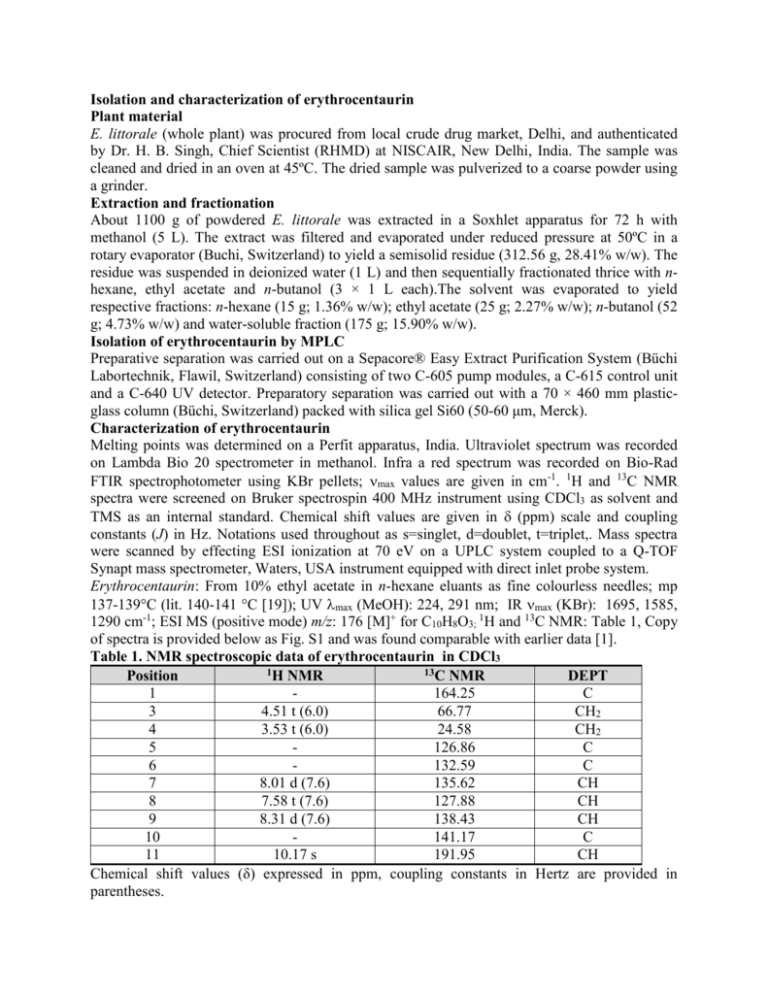
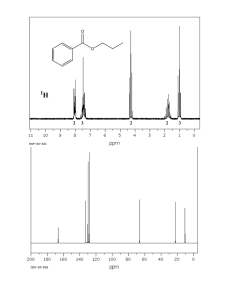
Isolation and characterization of erythrocentaurin Plant material E. littorale (whole plant) was procured from local crude drug market, Delhi, and authenticated by Dr. H. B. Singh, Chief Scientist (RHMD) at NISCAIR, New Delhi, India. The sample was cleaned and dried in an oven at 45ºC. The dried sample was pulverized to a coarse powder using a grinder. Extraction and fractionation About 1100 g of powdered E. littorale was extracted in a Soxhlet apparatus for 72 h with methanol (5 L). The extract was filtered and evaporated under reduced pressure at 50ºC in a rotary evaporator (Buchi, Switzerland) to yield a semisolid residue (312.56 g, 28.41% w/w). The residue was suspended in deionized water (1 L) and then sequentially fractionated thrice with nhexane, ethyl acetate and n-butanol (3 × 1 L each).The solvent was evaporated to yield respective fractions: n-hexane (15 g; 1.36% w/w); ethyl acetate (25 g; 2.27% w/w); n-butanol (52 g; 4.73% w/w) and water-soluble fraction (175 g; 15.90% w/w). Isolation of erythrocentaurin by MPLC Preparative separation was carried out on a Sepacore® Easy Extract Purification System (Büchi Labortechnik, Flawil, Switzerland) consisting of two C-605 pump modules, a C-615 control unit and a C-640 UV detector. Preparatory separation was carried out with a 70 × 460 mm plasticglass column (Büchi, Switzerland) packed with silica gel Si60 (50-60 μm, Merck). Characterization of erythrocentaurin Melting points was determined on a Perfit apparatus, India. Ultraviolet spectrum was recorded on Lambda Bio 20 spectrometer in methanol. Infra a red spectrum was recorded on Bio-Rad FTIR spectrophotometer using KBr pellets; max values are given in cm-1. 1H and 13C NMR spectra were screened on Bruker spectrospin 400 MHz instrument using CDCl3 as solvent and TMS as an internal standard. Chemical shift values are given in (ppm) scale and coupling constants (J) in Hz. Notations used throughout as s=singlet, d=doublet, t=triplet,. Mass spectra were scanned by effecting ESI ionization at 70 eV on a UPLC system coupled to a Q-TOF Synapt mass spectrometer, Waters, USA instrument equipped with direct inlet probe system. Erythrocentaurin: From 10% ethyl acetate in n-hexane eluants as fine colourless needles; mp 137-139°C (lit. 140-141 °C [19]); UV max (MeOH): 224, 291 nm; IR max (KBr): 1695, 1585, 1290 cm-1; ESI MS (positive mode) m/z: 176 [M]+ for C10H8O3; 1H and 13C NMR: Table 1, Copy of spectra is provided below as Fig. S1 and was found comparable with earlier data [1]. Table 1. NMR spectroscopic data of erythrocentaurin in CDCl3 1H NMR 13C NMR Position DEPT 1 164.25 C 3 4.51 t (6.0) 66.77 CH2 4 3.53 t (6.0) 24.58 CH2 5 126.86 C 6 132.59 C 7 8.01 d (7.6) 135.62 CH 8 7.58 t (7.6) 127.88 CH 9 8.31 d (7.6) 138.43 CH 10 141.17 C 11 10.17 s 191.95 CH Chemical shift values (δ) expressed in ppm, coupling constants in Hertz are provided in parentheses. Figure 1S. Copy of 1H and 13C NMR spectra of erythrocentaurin