Electroplating
advertisement

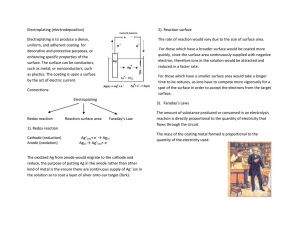
By Tyler Hanse Deposition of a thin layer of metal on a surface by an electrical process involving oxidation-reduction Chromium Chromium is used to coat steel parts on cars as well as other machines and steel products. This chrome plating helps to prevent corrosion and rusting. Nickel is used in the coating of several metal products, including our currency, many coins have been plated with nickel for protection from corrosion The reaction for plating nickel can be written as Ni2+ (aq) + 2e- Ni(s) Electroplating requires a power source usually a battery. The power source is supplies electrons to the cathode (the place where the reduction occurs). The electrons are created in the anode (where oxidation occurs) they then travel up the anode to the power source where they are propelled to the cathode and applied to the product that is being electroplated. Electroplating is similar to what we studied earlier in class, because it uses… Oxidation Reduction The transfer of electrons What is electroplating? Give two examples of how it is used. How does electroplating happen?