Electrochemical cell
advertisement

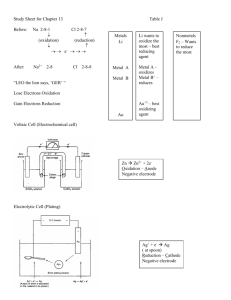
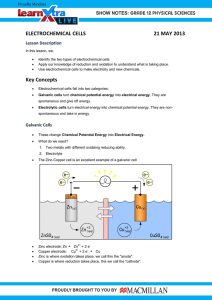
Electrochemical cell • Parts of a Voltaic Cell • The electrochemical cell is actually composed to two half cells. Each half cell consists of one conducting electrode ( a solid ) and a conducting solution. Each electrochemical cell consists of two solid conductors we call electrodes • Oxidation occurs in one half cell this produces electrons. • Reduction occurs in the other half cell this accepts electrons. • When the two half cells, consisting of a metal electrode and a conducting solution are connected with an external wire, the strongest oxidizing agent will undergo a reduction in one half cell and the strongest reducing agent will undergo an oxidation in the other half cell. Key Points 1) Cathode: The electrode (solid) at which reduction occurs The charge on the cathode is positive 2) Anode: The electrode (solid) at which the oxidation occurs The charge on the anode is negative , because it producing electrons 3) Direction of current flow: The electrons produced at the anode travel along the external wire to the cathode. 4) Spontaneity: The reaction in an electrochemical cell (also called a voltaic cell) is always spontaneous. 5) Ion flow: In the operating electrochemical cell, positive ions (cations) in the conducting solutions migrate toward the collection of electrons on the positive electrode or cathode, while negative ions migrates (move) toward the negative electrode or anode . Voltaic Cells Anode: negative Cathode: positive Voltaic cells: Electrochemical cells in which a spontaneous redox reaction can be harnessed to produce an electric current. Oxidation occurs at the anode Reduction occurs at the cathode The electrons are forced to flow through a wire by separating the reducing agent from the oxidizing agent. Calculating Voltages a) Locate the two half cells reactions in the table of standard reduction potentials. b) The half reaction that has the higher reduction potential will reduce and can be written as you find it in the table. c) The half reaction that has the lower reduction potential must be reversed and written as an oxidation. d) The E0 value of the higher half reaction is recorded unchanged from the table. e) The sign of the E0 value of the lower half reaction is changed and its value recorded. f) The two half reactions are balanced for the number of electrons exchanged but the value of each E0 remains unchanged. g) The two half reactions are then added together and so are the E0 values. ( This value will always be positive for an electrochemical cell). determine its voltage Au (s) | Au 3+ (aq) ||Cu 2+ (aq) | Cu (s) Gold Copper 1) Gold is higher the table and is written as found in table. • Au 3+ (aq) + 3 e- ------> Au (s) E0 = + 1.50 V 2) The copper is lower so it's reaction is written as an oxidation (sign of E0 reversed ). • Cu (s) ------> Cu 2+ (aq) + 2 eE0 = -0.34 V 3) Equation is balanced for the exchange of electrons ( each needs six). 2 Au3+ (aq) + 6e- ----> 2 Au (s) +1.50 V 3 Cu (s) ---->3 Cu2+ (aq) + 6e- -0.34 V • Note that the size of the values for the E0 of the reactions are not changed (even though the equations are multiplied, E0 is not) 4) Add the two half reactions and the E0 values. Cross off the electrons 2 Au3+ (aq) + 6e- ----> 2 Au (s) 3 Cu (s) ---->3 Cu2+ (aq) + 6e___________________________________ 2 Au3+ (aq) + 3 Cu (s) --> 2 Au (s) + 3 Cu2+ (aq) E0 = 1.16 v Spontaneous because it is positive