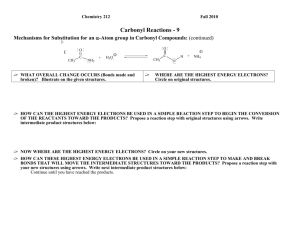
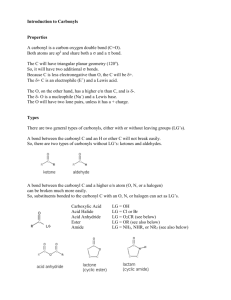
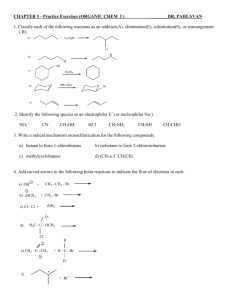
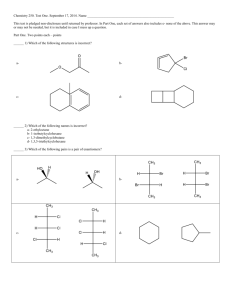
Carbonyl Compounds – Chapter 7 - Suggested answers to George
advertisement

Carbonyl Compounds – Chapter 7 - Suggested answers to George Facer p142-143
Q1. Ketones contain a C=O where the C is bonded to alkyl (or aryl groups), whereas in
aldehydes there is one alkyl (or aryl group) and one H bonded to the C of C=O.
2 CH3CH2CH2COCH3, CH3CH2COCH2CH3 and what I will now call “Dzatils molecule”
(CH3)2CHCOCH3 are the 3 possible molecules for C5H10O
3) A temp higher than the bpt of the aldehyde is needed to boil off the aldehyde for collection
while distilling. It not wanted that the alcohol boils off too (it would render the separation
attempt useless!) so a temp lower than the alcohol bpt is used to keep it (mostly) as a liquid
{you do get a small bit of alcohol vapour coming off}
4) In ethanal the hydrophobic part of the molecule is small and insignificant towards the
polarity of the molecule. In pentanal, the greatest proportion of the molecule is hydrophobic
hence it’s the polar CHO group that is insignificant, resulting in low solubility.
5) For molecules of the same (or very similar) numbers of electrons, hydrogen bonding is the
strongest of the intermolecular forces followed by dipole-dipole forces. Butan-1-ol therefore
has the strongest intermolecular forces and so the highest boiling point. Butanone contains a
dipole due to the polar C=O group but no hydrogen bonds resulting in its mpt being less than
that of butan-1-ol. Pentane has no hydrogen bonding and no dipole, but van der Waals forces
only so it has the lowest boiling point.
6) a) HCN is a gas at RT. The reaction would be HCN(g) + CH3COCH3(l)
CH3C(OH)(CN)CH3(l)
b) Given that Facer is now talking about alkali, I guess he means a solution based reaction
instead of the one in part a. In which case the reaction equation is:
HCN(aq) + CH3COCH3(l) CH3C(OH)(CN)CH3(l)
-
If no alkali is present, there will be no :CN nucleophile to attack the + C of the C=O. The
-
alkali makes the reaction progress as it deprotonates the weak acid HCN yielding the :CN
nucleophile.
7)
_
H
Li +
(1)
O
_
(2)
stage 1
O:
Al
H
H
H3C
H
dissolved in dry ether
CH3
H3C
H
O H
H + stage 2
CH3
H+ from
HCl(aq)
H3C
H
8) The reaction contains a single molecule, 2-hydroxypropanenitrile which cannot have a nonsuperimposable mirror image. The product is therefore not optically active.
9) This reaction produces molecules which are non-superimposable mirror images of each
other (i.e enantiomers), however, they are present in equal amounts because in their
–
formation, the :CN nucleophile had equal chance to attack either face of the flat ( trigonal
planar ) carbonyl. The rotation of the plane of rotation of monochromatic plane polarised light
from one enantiomer is cancelled by the other.
10) a) (NO2)2C6H3NHN=CHCH2CH3 obs: colourless propanal and orange solution produces an
orange/yellow precipitate.
CH3
b) CH3CH2COOH(aq) obs: colourless propanal added to a orange solution produces an
blue/green solution.
c) CH3CH2COO-(aq) colourless propanal and colourless Tollens reagent produce a silver mirror
on the side of a test tube on warming.
d) CH3CH2COO-(aq) colourless propanal and deep blue Fehlings reagent produce a red ppte
on warming.
e) No reaction. Not a methyl carbonyl. obs: colourless propanal is added to colourless mixture
of I2(aq) and NaOH(aq). No change occurs.
11) a )The formula C4H10O i.e. compound X, reveals there are no double bonds in the
molecule. Compound Y is an oxidation product of X which undergoes the iodoform test, so
must be a methly carbonyl. (CH3-C=O i.e. an acyl group must be present) The carbonyl
existence of Y is also seen from its reaction with 2,4-DNP. Y must be a ketone as it isn’t
oxidised by Fehlings reagent. Y must be CH3COCH2CH3 and X must be CH3CHOHCH2CH3.
b) Both would give a yellow precipitate.
c) CH3COCH2CH3 + NaOH(aq) + I2(aq) CHI3 + -OOCCH2CH3(aq) + Na+(aq)
balanced would be: CH3COCH2CH3 + 4 NaOH(aq) + 3 I2(aq) CHI3 + NaOOCCH2CH3(aq) +
3NaI(aq) + 3H2O(l)
d) Y must be a ketone so the alcohol it came from must be a 20 alcohol. It underwent the
iodoform reaction so it must be a methyl secondary alcohol.
H
H
H
OH
H
C
C
C
C
H
H
H
H
H
H
H
OH
H
C
C
C
H
H C H
H
H
H
butan-2-ol
Note: will 'do' triiodomethane reaciton
12
a) CH2=CHCH2CH2OH
a) CH3CH2CH2CH2OH
2-methylpropan-2-ol
Note: will NOT 'do' triiodomethane reaciton
13) There’s a suggestion in the Q (the two different boiling points), as to what is expected in
your answer i.e. distillation, but it is not necessary (see the second diagram). To play along
with the ‘clue’… First assemble the apparatus as shown in the diagram below.
Add a couple of anti-bumping granules and boil for about 20 minutes {ensure when heating
that the vapours do not progress too far up the condenser and do not use a Bunsen burner}.
After reaction, set up the apparatus for distillation to separate the butanone from any
unreacted butan-2-ol or any side products.
Thermometer
bulb level with
exit ‘arm’ of
still head
Open ‘gap’
shown
Collect the fraction that comes off at 80oC. The condensate will contain water. Add anhydrous
MgSO4 until clumping ends. Then separate using filtration. {Diagram should have clamp
positions on the neck of the round bottomed flask and half way along the condenser and
support at the bottom of the collection vessel.
Q14 a) Nucleophile: species with a pair of electrons which can be donated for the purpose of
forming a dative covalent bond. Oxidation is the loss of electrons, gain of oxygen or loss of
hydrogen for an atom in a given species.
b) i) nucleophilic addition.
ii) oxidation
iii) condensation reaction (or nucleophilic addition followed by elimination of water)
15. Recrystallise the orange precipitate by dissolving it in the minimum of hot solvent. Filter
when hot using fluted filter paper. Allow filtrate to cool slowly. Vacuum filter the crystals
using a Bucher funnel and aspirator. Wash crystals with a few drops of ice cold solvent. Allow
to dry in air (warm in oven if necessary). Put recrystallised crystals in a capillary tube to
about 2-3 mm in depth. Use melting point apparatus to determine the melting range. Take
the mid point to be the melting point value. Look up the value against reference tables for
2,4-DNP carbonyl derivatives.