pi-bonds-Carbonyl
advertisement
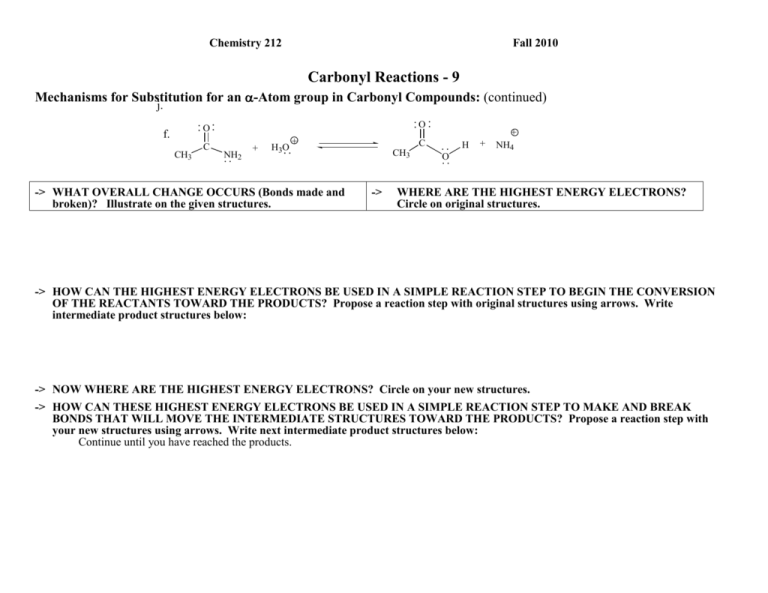
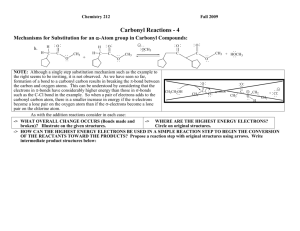
Chemistry 212 Fall 2010 Carbonyl Reactions - 9 Mechanisms for Substitution for an -Atom group in Carbonyl Compounds: (continued) j. O O f. CH3 C NH2 + H3O + -> WHAT OVERALL CHANGE OCCURS (Bonds made and broken)? Illustrate on the given structures. CH3 -> + C H O + NH4 WHERE ARE THE HIGHEST ENERGY ELECTRONS? Circle on original structures. -> HOW CAN THE HIGHEST ENERGY ELECTRONS BE USED IN A SIMPLE REACTION STEP TO BEGIN THE CONVERSION OF THE REACTANTS TOWARD THE PRODUCTS? Propose a reaction step with original structures using arrows. Write intermediate product structures below: -> NOW WHERE ARE THE HIGHEST ENERGY ELECTRONS? Circle on your new structures. -> HOW CAN THESE HIGHEST ENERGY ELECTRONS BE USED IN A SIMPLE REACTION STEP TO MAKE AND BREAK BONDS THAT WILL MOVE THE INTERMEDIATE STRUCTURES TOWARD THE PRODUCTS? Propose a reaction step with your new structures using arrows. Write next intermediate product structures below: Continue until you have reached the products. Carbonyl Reactions-9 2 Reflector’s Report Discussion: Identify the most important concepts you learned from this activity: What questions remain? Strategy Analyst’s Report Discussion: We had considerable discussion about identifying the HEE in reaction f. Using grammatically correct English sentences, present the final claim as to the tow possible sites for initial HEE and a warrant supporting the choice of the HEE used for the first reaction step. 3 Carbonyl Reactions-9 Out of Class Applications for Carbonyl reactions-9 A. Read: CGWW pp. 277-291, 292-294 & 723-735 B. Try to apply your mechanism(s) to example p of substitutions for carbonyl oxygen introduced in Carbonyl Rxns-1 and reproduced below. p. O O H3 O + H + OH + H2 O O O -> WHAT OVERALL CHANGE OCCURS? -> WHERE ARE THE HIGHEST ENERGY ELECTRONS? -> WHAT CAN THE HIGHEST ENERGY ELECTRONS DO TO AID IN THE NEEDED BOND MAKING AND BREAKING? -> NOW WHERE ARE THE HIGHEST ENERGY ELECTRONS? -> WHAT CAN THEY DO TO AID IN THE NEEDED BOND MAKING AND BREAKING? Continue until you have reached the products. C. Text Applications: Appropriate Questions: CGWW P. 302-: Problems 2, 4 & 6