Test One
advertisement
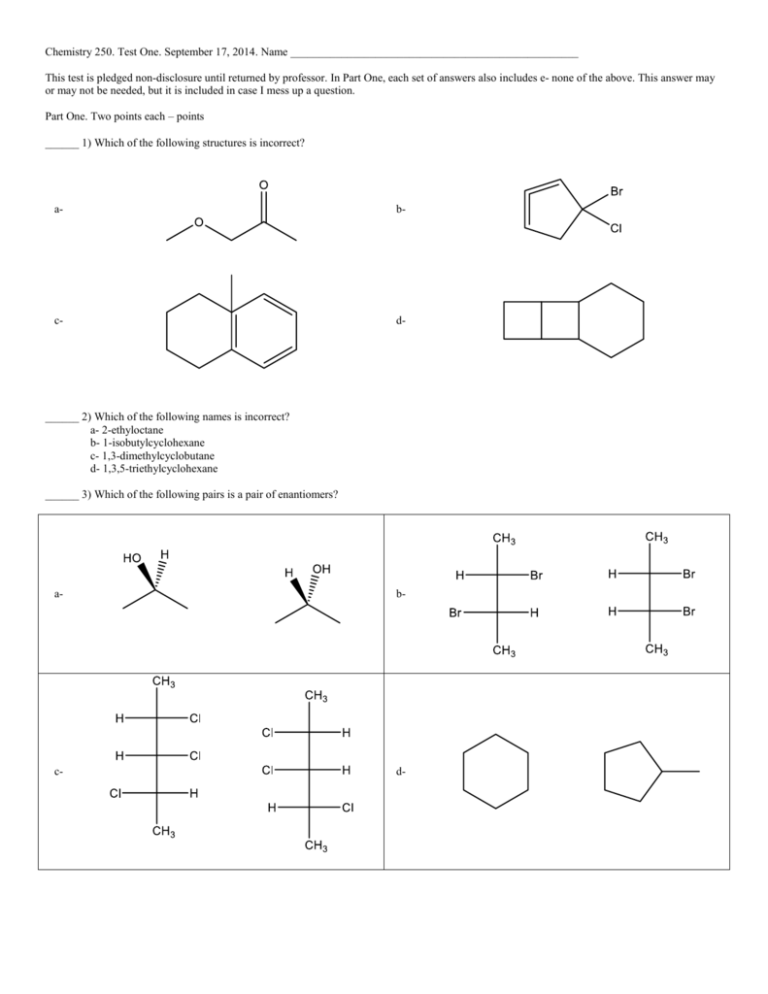
Chemistry 250. Test One. September 17, 2014. Name ___________________________________________________ This test is pledged non-disclosure until returned by professor. In Part One, each set of answers also includes e- none of the above. This answer may or may not be needed, but it is included in case I mess up a question. Part One. Two points each – points ______ 1) Which of the following structures is incorrect? a- b- c- d- ______ 2) Which of the following names is incorrect? a- 2-ethyloctane b- 1-isobutylcyclohexane c- 1,3-dimethylcyclobutane d- 1,3,5-triethylcyclohexane ______ 3) Which of the following pairs is a pair of enantiomers? a- b- c- d- ______ 4) Which of the following pairs is a pair of diastereomers? a- b- c- d- ______ 5) The following molecule has a standard rotation of -13.9o. What will be the rotation of its mirror image? a- -13.9o b- +13.9o c- cannot be determined d- will not rotate polarized light ______ 6) Which of the following molecules is a carboxylic acid? a- b- c- d- ______ 7) In methane, CH4, the molecule has what geometry and what are the bond angles? a- tetrahedral, 109.5o b- a square, 90o c- tetrahedral, 90o d- a planar triangle, 120o ______ 8) Which of the following trimethylcyclohexane rings is the most stable? a- b- c- d- ______ 9) Which of the following bondlines represents CH3CH2CH(CH3)C(CH3)2CH2CH2CH(CH3)2 a- b- c- d- ______ 10) The conformations of a given ring include an envelope form. The ring is a a- cyclobutane b- cyclopentane c- cycloheptane d- cyclohexane ______ 11) Which of the following is classified as cis? a- b- c- d- ______ 13) In the following molecule, how many quaternary, tertiary, and secondary carbons are there? a- 0 quaternary, 5 tertiary, 9 secondary b- 2 quaternary, 6 tertiary, 10 secondary c- 2 quaternary, 4 tertiary, 10 secondary d- 1 quaternary, 6 tertiary, 8 secondary Questions 14 and 15 apply to the following graph _____ 14) The Energy of Activation is shown by a- A b- B c- C d- D _____ 15) A transition state is shown by a- A b- B c- C d- D Part Two. 1) (8 points) Nomenclature a- b- c- d- 2) (9 points) Definitions – in words and complete. A drawing can help your answer, but alone it cannot get full credit. Substitution reaction – Leaving group - Intermediate in a reaction – 3) (10 points) Give the formula in proper (Hill) form, circle each functional group and give the name of the group. (Two molecules with three functional groups each) . Use 1,2,3 alcohols/amine/halides. Alkane is NOT used. 4) (6 points) Draw the three staggered conformers of 2,3-dimethylhexane in Newman projection of the C2-C3 bond. 5) (10 points) Draw and name the five structural isomers of C6H14. 6) (6 points) Circle all of the chiral carbons in the following molecule.