CHAPTER 5 - Practice Exercises (ORGANIC CHEM I ) ________DR
advertisement

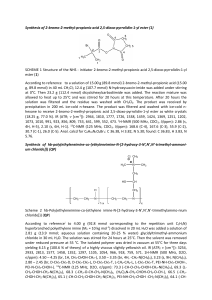
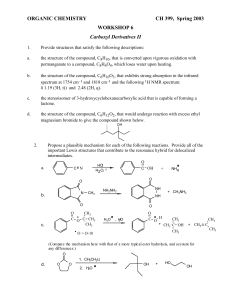
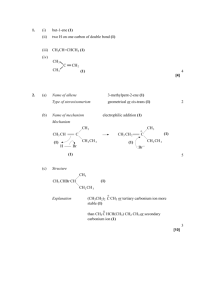
CHAPTER 5 - Practice Exercises (ORGANIC CHEM I ) ________DR. PAHLAVAN 1. Classify each of the following reactions as an addition(A), elimination(E), substitution(S), or rearrangement ( R). a) + Cl2/light Cl b) OH c) H2SO4 HBr /ether d) Br e) 2. Identify the following species as an electrophile( E+) or nucleophile( Nu:). NO2+ CN- CH3OH HCl CH3NH2 CH3SH CH3CHO 3. Write a radical mechanism monochlorication for the following compounds; a) butane to form 1-chlorobutane b) isobutane to form 2-chloroisobutane c) methylcyclobutane d) (CH3)3 C CH2CH3 4. Add curved arrows to the following polar reactions to indicate the flow of electrons in each. a) :OH CH3 - CH2 - Br + b) :OCH3 + CH3 - Br :NH3 c) Cl - Cl + .. O: .. d) H3C - C - OCH3 Cl .. O: e) CH3 - C - CH2 .. H + H - C - Br H f) + :Br 5. Using average bond enthalpies table , calculate Ho for the following reactions; CH3 - NH2 + H-Cl a) CH3 - Cl + H - NH2 b) CH4 + Br2 CH3 Br + HBr c) CH3 - CH2 - O - CH3 + HI CH3 - CH2 - OH + CH3 I 6. Give an example of each of the following; a) A nucleophile b) An electrophile c) A polar reaction d) a substitution reaction e) A heterolytic bond breaking f) A homolytic bond breaking g) A heterolytic bond forming h) A homolytic bond forming 7. Reaction of 2-methylpropene with HBr ,might in principle , lead to a mixture of two alkyl halide addition products. Name them, and draw their structures. 8. Complete the following reactions; a) Write the polar mechanism of the reaction of 1-methylcyclohexene with HCl, giving 1-chloro-1- methylcyclohexane as the product. b) Write the polar mechanism of the reaction of cyclohexene with Cl2, giving trans-1,2-dichlorocyclihexane. 9. Sketch a reaction diagram for the following reactions, label the parts of the diagram corresponding to reactants, products, transition states, Go , G # , and intermediate (if any). Also indicate if Go is positive or negative for each reaction. a) a one-step reaction which is fast and highly exergonic b) a two –step reaction with an endergonic first-step and an exergonic second step. 10. Look at the reaction energy diagram shown; E a) a) b) c) Is Go for the reaction positive or negative? Reaction progress How many steps are involved in the reaction? How many transition states are there? Label them on the diagram. Does reaction has an intermediate? Label it on the diagram. 11. Draw a reaction energy diagram for the overall reaction of propene with HBr. Two separate steps are involved, each with its own transition state.