Work-Sheet 8 - Chemistry With BT
advertisement

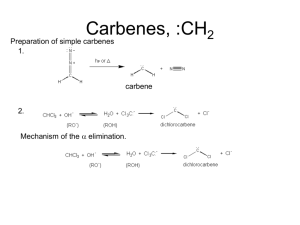
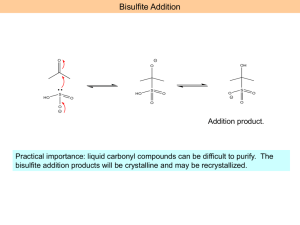
ORGANIC CHEMISTRY II WORKSHEET 8 Week 10 The carbonyl group is polarized in such a way that the -electron density is concentrated around the oxygen atom, thus making the carbon atom electron deficient. This can be visualized using resonance theory. Please write a resonance structure for a generic carbonyl group to validate the statement above: C O ? It is clear from the discussion above that a carbonyl carbon is susceptible to nucleophilic attack. This can happen in both acid and base catalyzed conditions. In order to fully understand acid-catalyzed conditions, we need to realize that the carbonyl oxygen is a Lewis base and can undergo protonation easily. Illustrate the protonation of a carbonyl oxygen below: C O + H ? Now show each step of an acid catalyzed hydration reaction: First step is an acid base reaction as above Second step is attack of water to the protonated carbonyl C O + H3O ? Why don’t we apply what we learned here to a slightly different case… Still same two steps as above; but the nucleophile is slightly different than water. O H H + CH3CH2OH ? (1 equivalent) What is the new functional group formed called? Can the product be isolated? Write a full mechanism for the reaction of benzaldehyde with two equivalents of ethanol under acid catalyzed conditions. What is the new functional group formed called? Under what conditions can the product be isolated? Under certain reaction conditions the product of the reaction below can be isolated, when benzaldehyde is treated with only one equivalent of alcohol. Write a full mechanism to explain how this might happen. O H H + HO ? OH (1 equivalent) The reaction below illustrates the hydration of benzaldehyde under base-catalyzed conditions. Complete the reaction. O H + OH ? The reaction product between benzaldehyde and another carbon nucleophile is given below. The formation of alcohol from the alkoxide is generally referred to as the “work-up” step. Show the details of the “workup” step. O OH H + Na C C CH3 Sometimes it is possible that there are more than one electrophilic sites in a molecule. Identify the electrophilic carbons in the following molecule. O Br H Write down the reaction products obtained when the molecule above is treated with the following acetylide Na C C CH3 Clearly, both electrophilic sites are susceptible to nucleophilic attack by the acetylide. How would you be able to direct the nucleophile to only one of the electrophilic sites? Assume that you wish to synthesize the molecule below: O H It is possible to deactivate the aldehyde functionality temporarily, and obtain the aldehyde back after the benzylic carbon is functionalized. This strategy is referred to as “protection” in synthetic organic chemistry. You can utilize a method you worked out at the beginning of the worksheet in order to “protect” aldehydes and ketones. Outline a synthetic strategy to prepare the target molecule above. Protection group strategy has numerous applications in synthetic organic chemistry. How about using both Grignard and protecting group strategy to synthesize the deuterated benzaldehyde below? O O H Br H D What would happen if you used Grignard strategy without adequately protecting the aldehyde? How about a slight variation on the same theme? O HO O + Br O