Periodic Table of The Elements
advertisement
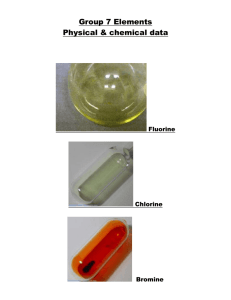
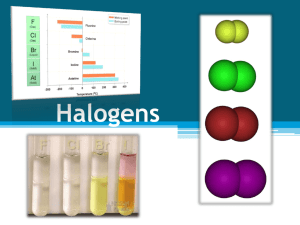
Periodic Table of The Elements http://www.chemicalelements.com Group VII – The Halogens The halogens are five non-metallic elements found in group 7 of the periodic table. The term "halogen" means "salt-former" and compounds containing halogens are called "salts". All halogens have 7 electrons in their outer shells, giving them an oxidation number of -1. The halogens exist, at room temperature, in all three states of matter Solids: Iodine and Astatine Liquids: Bromine Gases: Fluorine and Chlorine Basic Information Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point: -219.62 °C (53.530006 °K, 363.31598 °F) Boiling Point: -188.14 °C (85.01 °K, -306.652 °F) Number of Protons/Electrons: 9 Number of Neutrons: 10 Classification: Halogen Crystal Structure: Cubic Density @ 293 K: 1.696 g/cm3 Color: Greenish Bentor, Yinon. Chemical Element.com - Fluorine. Jun. 3, 2002 <http://www.chemicalelements.com/elements/f.html>. Basic Information Bentor, Yinon. Chemical Element.com Chlorine. Jun. 3, 2002 <http://www.chemicalelements.com/eleme nts/cl.html>. Name: Chlorine Symbol: Cl Atomic Number: 17 Atomic Mass: 35.4527 amu Melting Point: -100.98 °C (172.17 °K, 149.764 °F) Boiling Point: -34.6 °C (238.55 °K, 30.279997 °F) Number of Protons/Electrons: 17 Number of Neutrons: 18 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.214 g/cm3 Color: green Facts about Bromine: 1. Bromine is a red liquid at room temperature. 2. It is only one of three liquid elements at room temperature. 3. It is abundant in the ocean. 4. It was discovered by Antoine J. Balard Bentor, Yinon. Chemical Element.com - Bromine. Jun. 3, 2002 <http://www.chemicalelements.com/elements/br.h tml>. 5.The name of the elements is from the Greek word bromos (stench) Iodine is a bluish-black, lustrous solid. It evaporates at room temperatures into a pretty blue-violet gas with an irritating odor. It forms compounds with most elements, but is less reactive than the other halogens, which displace it from iodides. Iodine exhibits some metallic-like properties. It dissolves readily in chloroform, carbon tetrachloride, or carbon disulfide to form beautiful purple solutions. It is only slightly soluble in water. Iodine compounds are important in organic chemistry and very useful in medicine and photography. Lack of iodine is the cause of goitre. The deep blue colour with starch solution is characteristic of the free element. It is used by seaweeds from which it may be recovered, and is found in Chilean saltpeter, caliche, old salt brines, and salt wells. http://webelements.com as·ta·tine (1947): a radioactive halogen element discovered by bombarding bismuth with alpha particles and also formed by radioactive decay Bentor, Yinon. Chemical Element.com - Astatine. Jun. 3, 2002 <http://www.chemicalelements.com/elements/at.html>. Facts Date of Discovery: 1940 Discoverer: D.R. Corson Name Origin: From the Greek word astatos (unstable) Uses: No uses known Obtained From: Man-made