Level 3: practice Version 3Prep/Intervention
advertisement
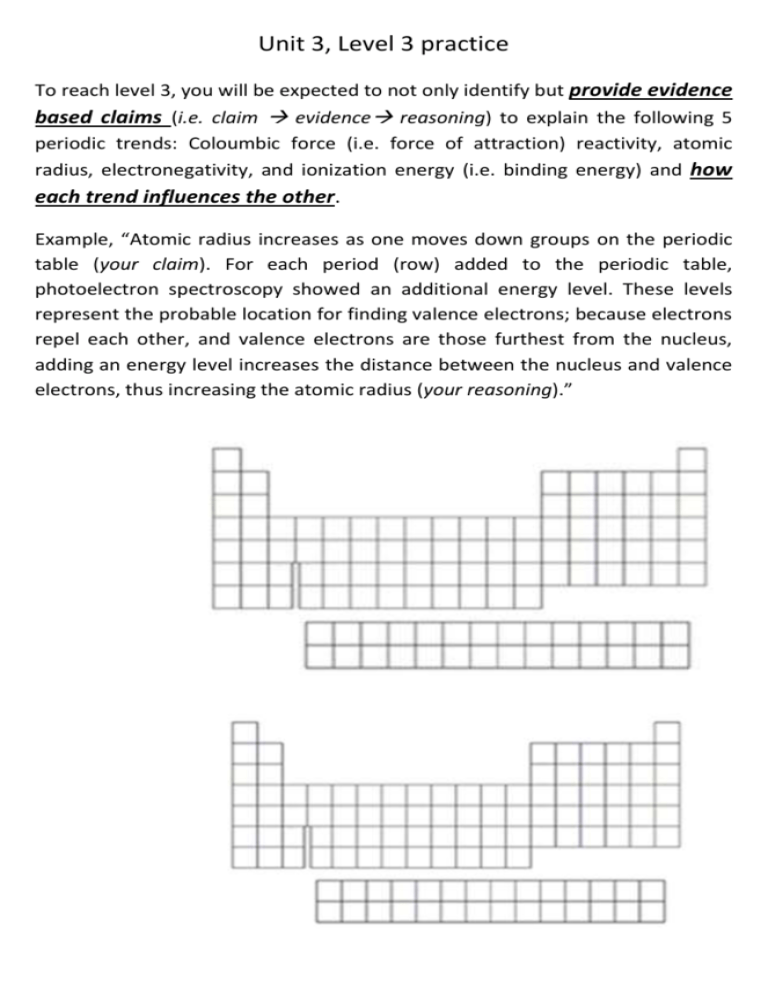
Unit 3, Level 3 practice To reach level 3, you will be expected to not only identify but provide evidence based claims (i.e. claim evidence reasoning) to explain the following 5 periodic trends: Coloumbic force (i.e. force of attraction) reactivity, atomic radius, electronegativity, and ionization energy (i.e. binding energy) and how each trend influences the other. Example, “Atomic radius increases as one moves down groups on the periodic table (your claim). For each period (row) added to the periodic table, photoelectron spectroscopy showed an additional energy level. These levels represent the probable location for finding valence electrons; because electrons repel each other, and valence electrons are those furthest from the nucleus, adding an energy level increases the distance between the nucleus and valence electrons, thus increasing the atomic radius (your reasoning).”