Lewis Dot Diagrams: Chemistry Presentation
advertisement
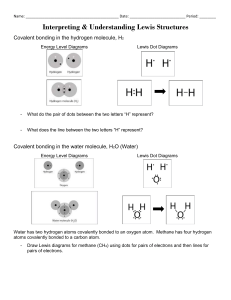
Lewis Dot Diagrams • Each row of the periodic table is called a period • All elements in a period have the same number of atomic orbitals (or shells) • Each column of the periodic table is called a group or family • All elements in a group have the same number of valence electrons Bohr Diagram Review • Draw the Bohr diagram for the following: Oxygen, Fluorine, Sodium Lewis Dot Diagrams •Method of representing atoms •Discovered by Gilbert N. Lewis •In a dot diagram, only the symbol for the element and the electrons in its outermost energy level (valence electrons) are shown • Eg: Sodium has 1 valence e- in it’s outermost shell, the diagram for sodium would look like this Na Drawing Lewis Dot diagrams in 3 easy steps Getting Started: 1. Find your element on the periodic table. 2. Determine the number of valence electrons. -this will be determined by which group your element is in (eg: Li is in group 1, so it has 1 valence electrons) 3. This is how many electrons you will draw. • Lone pair – electrons in atom’s valence shell (not used in bonding) ex: Na • Bonding pair – electron pair involved in bonding ex: N Example 1 - Carbon 1. Write the symbol for the element. 2. How many valence electrons does it have? 3. Start on the top and draw the electrons clockwise or counterclockwise around the element . 4. Check your work— did you draw the correct number of electrons? C Example 2 - Chlorine Cl Your Turn • Draw Lewis diagrams for the following: Magnesium, Neon and Boron Mg Ne B