You saw in Chapter 5 that early scholars imagined a universe made
advertisement

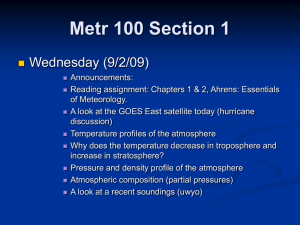
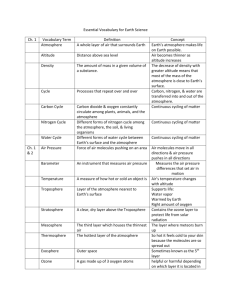
Instructions: Read the text below. This is obviously a terrible way to present this text for a reader. With a small group you will decide what text features should be added to make this text easier to understand. You must give this text a title – and 2 subheadings. Decide what words you would put in boldface. What pictures, diagrams, charts, graphs, and sidebars/textboxes would you use? On a larger piece of paper you will set up a rough design for your text, that shows were all text features will be located. You saw in Chapter 5 that early scholars imagined a universe made of four basic “elements”: air, water, earth, and fire. We now know that fire refers only to the energy given off by burning, so it is not made of matter. Air, water and earth are made of matter, but they are not elements in the scientific sense. In this section, you will look more closely at these three substances to see what chemical elements each contains. The term atmosphere usually refers to a gaseous envelope surrounding a planet. Nitrogen, oxygen, argon, and carbon dioxide are part of the composition that makes up Earth’s atmosphere. Three of these gases go through interesting cycles that are essential to life on our planet. A cycle is a “return trip” for a substance. It goes through a sequence of steps or processes and then comes back to its starting point. You may also remember these cycles from your earlier science studies. Oxygen is so reactive that it cannot exist for more than a fleeting instant as an uncombined atom. The oxygen in the atmosphere is therefore in the form of diatomic O2 molecules. Nearly all living cells depend on oxygen to release energy through cell respiration. The nitrogen gas in the atmosphere consists of N2 molecules. This element is vital to life. Every protein in your body contains nitrogen atoms that cycled through the atmosphere at some time. Gaseous argon consists of single, uncombined Ar atoms. Argon is unreactive and is not involved in a cycle. The carbon in our atmosphere is present in carbon dioxide (CO2) molecules, which also contain the element oxygen. Plants and plant-like organisms use CO2 to make the food we depend on and then release O2 back to the atmosphere. Dry air does not contain any hydrogen at all. However, air is seldom truly dry. It contains a variable percentage of water vapour – more on a hot humid day above a lake, less above a desert. Thus air does contain the element hydrogen in H 2O molecules. These molecules are cycled back and forth between the atmosphere and Earth. Earth is sometimes called “the big blue marble” because water dominates its surface. Even land that seems dry may have water lying or flowing beneath it. Much of what appears to be land in Canada’s North is actually “sea ice”: solid water floating on the Arctic Ocean. Together, all of this water makes up the hydrosphere, a nearly continuous layer of water lying on or just under Earth’s crust.