Chemical Kinetics Worksheet: First-Order Reactions & Ea
advertisement
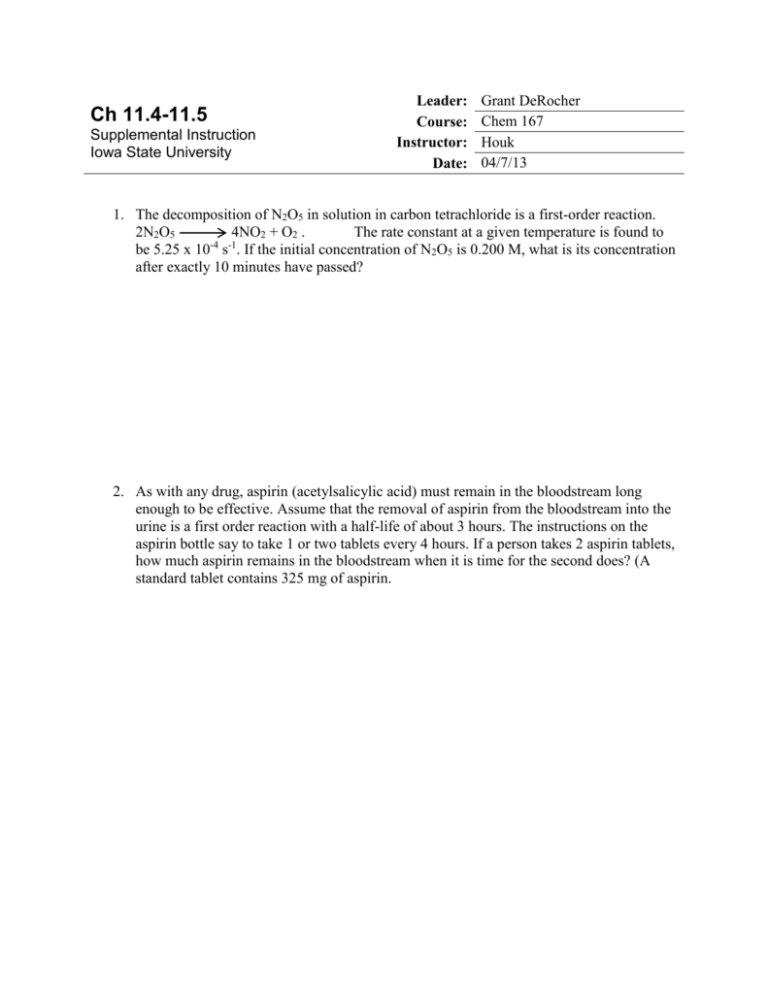
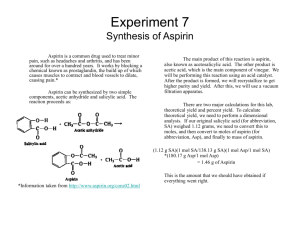
Ch 11.4-11.5 Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Grant DeRocher Chem 167 Houk 04/7/13 1. The decomposition of N2O5 in solution in carbon tetrachloride is a first-order reaction. 2N2O5 4NO2 + O2 . The rate constant at a given temperature is found to be 5.25 x 10-4 s-1. If the initial concentration of N2O5 is 0.200 M, what is its concentration after exactly 10 minutes have passed? 2. As with any drug, aspirin (acetylsalicylic acid) must remain in the bloodstream long enough to be effective. Assume that the removal of aspirin from the bloodstream into the urine is a first order reaction with a half-life of about 3 hours. The instructions on the aspirin bottle say to take 1 or two tablets every 4 hours. If a person takes 2 aspirin tablets, how much aspirin remains in the bloodstream when it is time for the second does? (A standard tablet contains 325 mg of aspirin. 3. If the initial concentration of the reactant in a first-order reaction A products is 0.64 mol/L and the half-life is 30.0 s, (a) Calculate the concentration of the reactant exactly 60 s after initiation of the reaction. (b) How long would it take for the concentration of the reactant to drop to one-eighth its initial value? (c) How long would it take for the concentration of the reactant to drop to 0.040 mol/L? 4. The activation energy for the reaction in which CO2 decomposes to CO and a free radical oxygen atom, O, has an activation energy of 460 kJ/mol. The frequency factor is 2x1011 s-1. What is the rate constant for this reaction at 298 K? 5. The following rate constants were obtained in an experiment in which the decomposition of gaseous N2O5 was studied as a function of temperature. The products were NO2 and NO3. k(s-1) Temperature (K) 298 3.4 x 10-5 308 2.2 x 10-4 318 6.8 x 10-4 328 3.1 x 10-3 Determine Ea for this reaction in kJ/mol. 6. The table below presents rate constants measured at three temperatures for the following reaction, which is involved in the production of nitrogen oxides in internal combustion engines. O(g) + N2(g) NO(g) + N(g) k(L/(mol*s)) 4.4 x 102 2.5 x 105 5.9 x 106 Temperature (K) 2000 3000 4000 Determine the activation energy of the reaction in kJ/mol.