Floral Park Memorial High School - Sewanhaka Central High School
advertisement

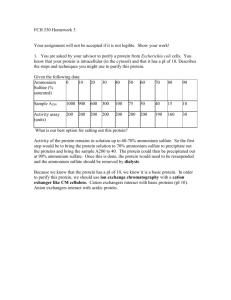
Floral Park Memorial High School The Sewanhaka Central High School District NAME:_____________________________________ DATE:________________________________ Mrs. Naus Forensic Science Chapter 5 Take Home Exam Directions:Place all of your answers IN PEN on the exam answer sheet at the end 1- Any type of matter that does NOT contain the element carbon is considered: 2-Where in the periodic table are the heaviest elements located? 3- A”Fingerprint “ of a paint sample can be made by the process of ________. 4- When separating black ink in the process of paper chromatography, the different colored bands are created by the different _________________ of the molecules. 5- A “fingerprint” of a substance such as heroin can be created through the process of: write out the letter and answer on your answer sheet a- gas chromatography b- gel electrophoresis c- infrared spectrogram d- light dispersion 6- Definition: the simplest substance known provides the building blocks for all matter. Use the picture below to answer questions 7-9 Picture A Picture B Picture C 7- Picture C: write out the letter and answer on your answer key A- has definite shape and definite volume B- has no definite shape but takes on the volume of its container C- has no definite shape or volume D- has definite volume but takes on the shape of its container 8- The atomic arrangement of a gas is most likely represented by which picture? 9- Identify the phase of matter and define its shape and volume for Picture B. 10- A charged atom is defined as:_____________ Use the scenerio below to answer questions 11 and 12 A dead fisherman is found floating belly up down the east river. A vile of white liquid is found in his pocket. After performing an infrared spectrogram the liquid is determined to be 92% rat poison and 8% ether. 11- What is the qualitative conclusion of this substance? 12- What is the quantitative conclusion of this substance? 13- In the chemical reaction between sodium (Na) and chloride (Cl), sodium gives up an electron to chloride therefore forming ___________________. Write both the letter and the answer on your answer sheet A- atom B- element C- ion D- solid 14- Which phase change changes a solid to a gas? 15- _____________________ is defined as the ability to do work. 16- All methods of chromatography contain both a _____________ and _______________ phase. 17- IN YOUR OWN WORDS, DEFINE CHROMATOGRAPHY. Use the picture below to answer questions 18 and 19 Solvent front Ether Rat Poison Origin 1 2 3 18- As the solvent moves through the paper, which solute dissolves easier and explain how you know this. 19- Can you identify the contents of the unknown sample displayed in column 3 and if so explain how. 20- List the four properties used to separate a solute in the process of chromatography. 21- Before injecting a sample into a gas chromatography column it must be heated or __________________ in order to make it a __________________. 22-Solutes that display polarity and interact with the stationary portion in an HPLC column have: Write your letter choice and answer on your answer sheet A- A smaller Rf value due to the distance they have to travel B- A smaller retention time due to the size of the molecules C- A smaller retention time but a greater Rf value D- A greater retention time due to the speed of the molecules Use the gas chromatogram below to answer questions 23-25 23- Qualitative analysis of Methane is determined by its: Write out the letter and answer on your answer key A- Peak height B- retention time C- peak width D- Choices A and C NOT B 24- Which solute is most soluble in this chromatogram and how do you know? What I mean is which molecules reacted best with the gas and how do you know? 25- Explain how you quantitatively determine the concentration of each substance. 26- Explain how Thin Layer chromatography is similar to paper chromatography. 27- Define Rf value and explain when it is used. 28- As you move from gamma to radio rays, the wavelength (increases or decreases) while the frequency of wavelengths (increase or decrease) 29- Which type of chromatography takes advantage of the ability to change the temperature? 30- the function of restriction enzymes are to: Write out the letter and the answer on your answer sheet. A- to separate DNA fragments based on size and solubility B- to locate and cut specific sequences of DNA C- to cut a polypeptide into single amino acids D- none of the above 31- Which process is commonly used to identify blood, proteins, DNA and enzymes? 32- After white light is dispersed through a prism, which color has the largest wavelength? 33- The only property different in each part of the electromagnetic spectrum is: A- wavelength B- light absorption C- frequency D- spectrogram Use the diagram below to answer questions 34and 35 34- Label each part of this blood sample. 1 35- Which part is most important to gel electrophoresis and why? 2 3 36- The fragments that migrate quickest towards the positive end of an electrophoresis are: Write out the letter and answer on your answer sheet A- small and positive B- large and positive C- large and negative D- small and negative Use the gel electrophoresis below to answer questions 37-40 Examine the diagram of an agarose gel below and answer the following questions. 37-What do the bands in the drawing represent? 38-Which bands traveled the slowest? (use the number) 39- If band A is found on the victim and bands B-E are the suspects can any of these people be found guilty? Why or why not? 40-What causes the fragments to move through the gel?