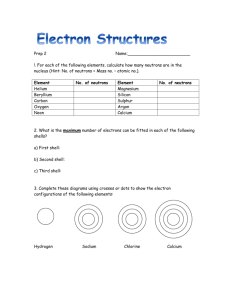
CHEM 5013
advertisement
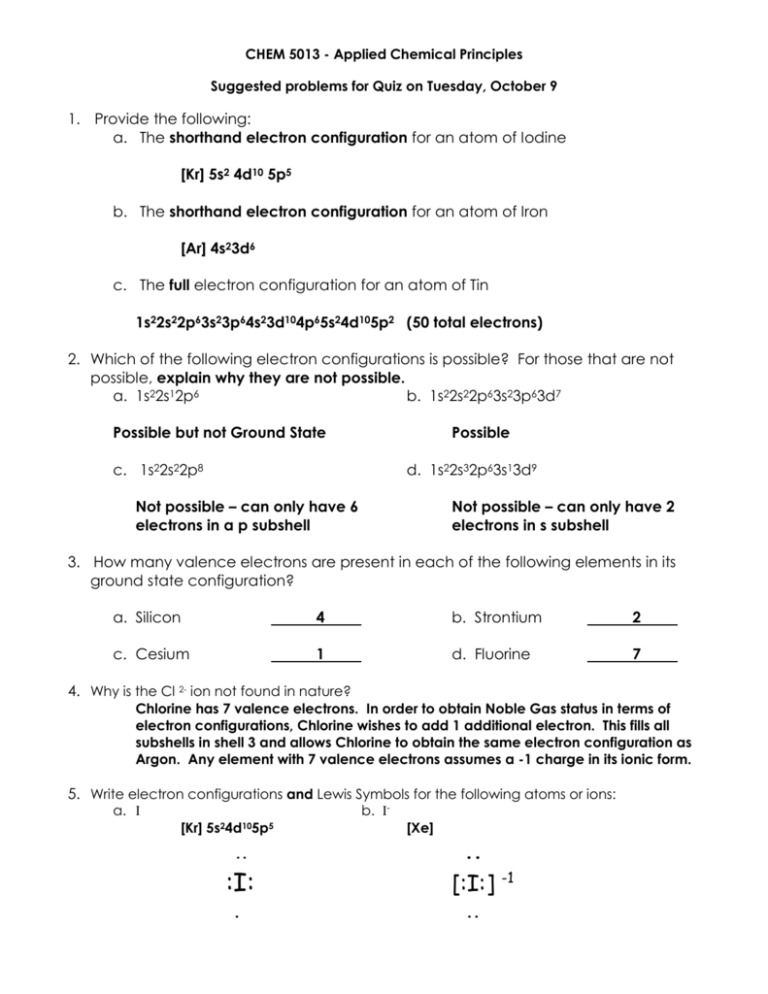
CHEM 5013 - Applied Chemical Principles Suggested problems for Quiz on Tuesday, October 9 1. Provide the following: a. The shorthand electron configuration for an atom of Iodine [Kr] 5s2 4d10 5p5 b. The shorthand electron configuration for an atom of Iron [Ar] 4s23d6 c. The full electron configuration for an atom of Tin 1s22s22p63s23p64s23d104p65s24d105p2 (50 total electrons) 2. Which of the following electron configurations is possible? For those that are not possible, explain why they are not possible. a. 1s22s12p6 b. 1s22s22p63s23p63d7 Possible but not Ground State Possible c. 1s22s22p8 d. 1s22s32p63s13d9 Not possible – can only have 6 electrons in a p subshell Not possible – can only have 2 electrons in s subshell 3. How many valence electrons are present in each of the following elements in its ground state configuration? a. Silicon 4 b. Strontium 2 c. Cesium 1 d. Fluorine 7 4. Why is the Cl 2- ion not found in nature? Chlorine has 7 valence electrons. In order to obtain Noble Gas status in terms of electron configurations, Chlorine wishes to add 1 additional electron. This fills all subshells in shell 3 and allows Chlorine to obtain the same electron configuration as Argon. Any element with 7 valence electrons assumes a -1 charge in its ionic form. 5. Write electron configurations and Lewis Symbols for the following atoms or ions: a. I b. I- [Kr] 5s24d105p5 .. :I: . [Xe] .. [:I:] -1 .. c. Ba d. Ba2+ 6s2 [Xe] [Xe] . Ba . [Ba]2+ 6. Use Lewis symbols to represent the transfer of electrons between Calcium and Sulfur to form Calcium Sulfide, composed of two ions each with noble-gas configuration. ▪ ▪ Ca ▪ + :S: .. [Ca]2+ + [ : S : ] ▪ 2- .. What is the chemical formula of the product, Calcium Sulfide? CaS 7. Use Lewis symbols to represent the transfer of electrons between Sodium and Oxygen to form Sodium Oxide. ▪ ▪ Na + : O : ▪ ▪ + Na .. 2 [Na] +1 + [ : O : ] .. What is the chemical formula of the product, Sodium Oxide? Na2O 2-