Electron Configuration & Rules: Chemistry Practice
advertisement
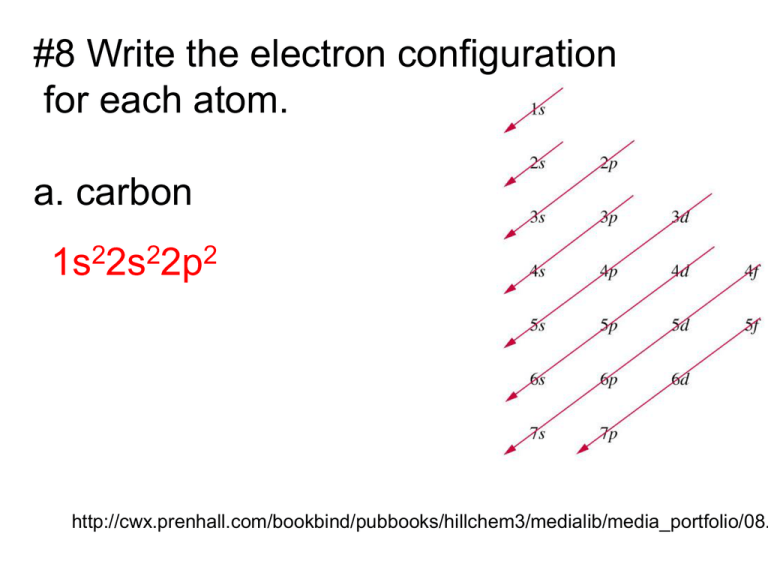
#8 Write the electron configuration for each atom. a. carbon 1s22s22p2 http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/08. #8b. argon argon has 18 electrons 1s22s22p63s23p6 #8c. nickel nickel has 28 electrons 2 2 6 2 6 2 8 1s 2s 2p 3s 3p 4s 3d or it can be written: 2 2 6 2 6 8 2 1s 2s 2p 3s 3p 3d 4s #9a. boron boron has 5 electrons 2 2 1 1s 2s 2p #9b. silicon silicon has 14 electrons 2 2 6 2 2 1s 2s 2p 3s 3p #10 aufbau principle Pauli exclusion principle Hund’s rule Aufbau Principle Think: “falling off the bow to the lowest bottom. (of the lake)” Link to Orbital Diagram Practice Page More Electron Configuration practice LINK Pauli exclusion principle Think: “exclusive dinner for only two” Hund’s Rule Think: “the Huns wanted more space” http://intro.chem.o #11 Explain why the actual electron configurations for some elements differ from those assigned using the aufbau principle. You can see it in this website (click on the pic. when filling the 3d orbitals) #12. Use fig 5.7 to arrange the following sublevels in order of decreasing energy 2p, 4s, 3s, 3d, 3p. 3d 4s 3p 3s 2p #13 Why does one electron in a potassium atom go into the fourth energy level instead of squeezing into the third energy level along with the eight already there? 2 2 6 2 6 1 1s 2s 2p 3s 3p 4s Because the sublevels, 3s and 3p are already filled. The next electron has to find a slightly higher energy level to go to.