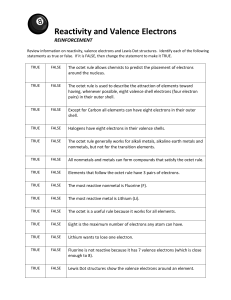
Electron Configuration & Octet Rule Worksheet
advertisement
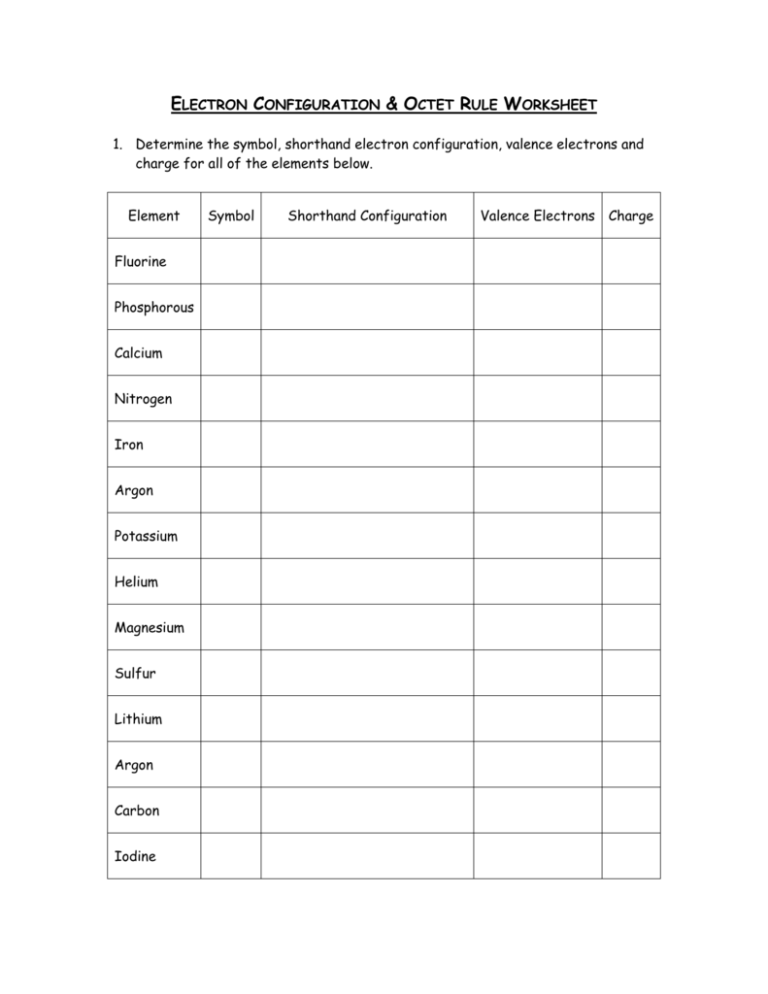
ELECTRON CONFIGURATION & OCTET RULE WORKSHEET 1. Determine the symbol, shorthand electron configuration, valence electrons and charge for all of the elements below. Element Fluorine Phosphorous Calcium Nitrogen Iron Argon Potassium Helium Magnesium Sulfur Lithium Argon Carbon Iodine Symbol Shorthand Configuration Valence Electrons Charge Oxygen Barium Aluminum Hydrogen Xenon Bismuth 2. Identify each of the following elements as a metal, nonmetal or semimetal and the group (by property) the element belongs to. o Sodium ____________________________________________ o Silicon ____________________________________________ o Neon ____________________________________________ o Calcium ____________________________________________ o Nitrogen ____________________________________________ o Iron ____________________________________________ o Lead ____________________________________________ o Uranium ____________________________________________ 3. State the octet rule. How is it used to predict ion charge?