Additional homework:
advertisement

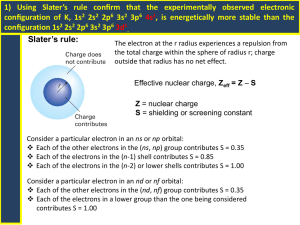
Homework (Textbook) Problems 1.3 Rb = [Kr]5s1, Ag = [Kr]4d105s1 Energy level of hydrogen atom is , If the electron in H atom is at 5S orbital, E5 = -B/25 = -13.6/25 = 0.55 eV. 1.4 Since , the energy of incident light = hv = hc/λ= 21.3 eV Kinetic energy of ejected electron from Kr = mv2/2= 7.1 eV. Kinetic energy of ejected electron from Rb = mv2/2= 17.1 eV. Therefore, ionization energies of Kr and Rb are 14.2 and 4.2 eV, respectively. 1.10 Electron configuration of Be is 1s22s2 and Z is 4. The reduction of the true nuclear charge to the effective nuclear charge is called shielding, Based on Slater’s rule: For the 2s perspective, S = 0.35*1 + 1*0.85 = 1.2, Zeff = 2.8. For the 1s perspective, S = 0.35, Zeff = 3.65. The electrons in 2S are shielded by core electrons, i.e 1s electrons. 1.12 dx2-y2 = (15/16π)1/2sin2θcos2φ and dxy = (15/16π)1/2sin2θsin2φ Additional homework: 1. (a) For g orbital, l is 4 and l = 0, 1, …, n-1. Therefore, the lowest n value is 5. (b) ml = 4, 3, 2, 1, 0, -1, -2, -3, -4. (c) 18 electrons. 2. Group IIa, i.e. Be, Mg, Ca, Sr, Ba, Ra group. 3. If wavefunction is in the form of y(x) = y0cos(kx) boundary condition for particle in the box, at x = 0, and x= L, y(0)= y(L) = 0 However, y(0) = y0cos(0)= y0 ≠0. Therefore, the wavefunction cannot be in the form of y(x) = y0cos(kx). 4. (a) Ca =[Ar]4S2, Ca2+ = [Ar] (b) Ti = [Ar]3d24S2, Ti2+ = [Ar]3d2 (c) Cr = [Ar] 3d44S2, Cr3+ = [Ar]3d3 (d) Cu = [Ar]3d104S1, Cu2+ = [Ar]3d9 5. (a) O-, Z = 8, (1s)2(2s, 2p)7, S(2p) = 6*0.35 + 0.85*2 = 3.8, Z* = 8 – 3.8 = 4.2 S-, Z = 16, (1s)2(2s, 2p)6(3s, 3p)7, S(3p) = 6*0.35 + 6*0.85 + 2*1 = 9.2, Z* = 16 – 9.2 = 6.8 Se-, Z = 34, (1s)2(s2, 2p)6(3s, 3p)6(3d)10(4s, 4p)7, S(4p) = 6*0.35 + 16*0.85 + 8*1 = 23.7, Z* = 34 – 23.7 = 10.3 For oxygen, E = E1(Z* 2 /n2)= 13.6(4.2)2/22 = 60.0 eV EA for O ~ 60.0 eV Similarly, EA for S ~77.2 eV, EA for Se ~ 90.2 eV. (b) The empirical EAs are O = 1.46 eV, S = 2.08 eV, Se = 2.02 eV. The general trend is EA decreases down the Group. Therefore, Slater's rule is far from correct. On the other hand, the EA for oxygen is extremely small due to the strong electron repulsion between electrons in a very small atom.