Prep 2 Electron structures
advertisement
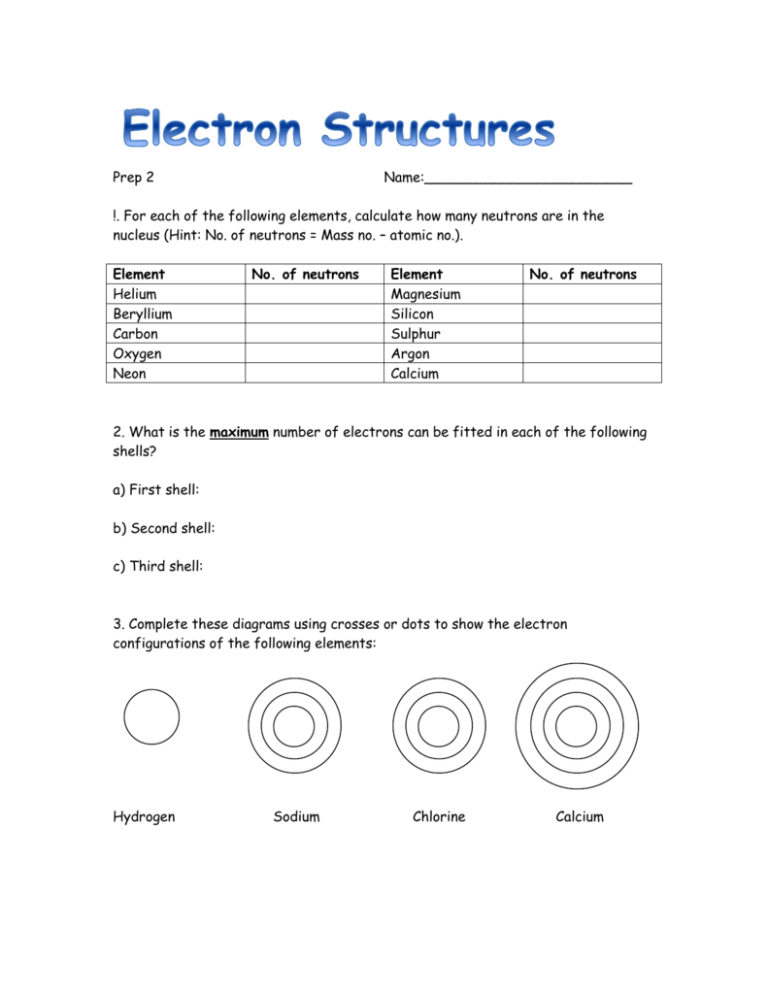
Prep 2 Name:________________________ !. For each of the following elements, calculate how many neutrons are in the nucleus (Hint: No. of neutrons = Mass no. – atomic no.). Element Helium Beryllium Carbon Oxygen Neon No. of neutrons Element Magnesium Silicon Sulphur Argon Calcium No. of neutrons 2. What is the maximum number of electrons can be fitted in each of the following shells? a) First shell: b) Second shell: c) Third shell: 3. Complete these diagrams using crosses or dots to show the electron configurations of the following elements: Hydrogen Sodium Chlorine Calcium 4. Write, using the number and comma notation (e.g. 2,8,1), the electron configurations of the first 10 elements in the Periodic Table. 5. How many electrons does sodium (Na) have in it’s outer shell? 6. What group of the Periodic Table is sodium in? 7. How many electrons does a sodium atom lose to have a full outer shell? 8. What would the new electron configuration be of a sodium atom that has lost its outer electron?











