Conservation Genetics Resources Electronic Supplementary M
advertisement

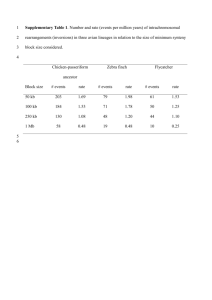
Conservation Genetics Resources Electronic Supplementary Material ESM_1. Additional details including an extended, quantitative descriptions of clone screening and 454-sequencing results, multiplex groups and the assignment of South Island robin microsatellite loci to homologous locations in the zebra finch genome. Isolation and characterisation of microsatellite markers from the South Island robin (Petroica australis) Sheena M. Townsend, Tania M. King and Ian G. Jamieson Allan Wilson Centre for Molecular Ecology and Evolution, Department of Zoology, University of Otago, 340 Great King Street, Dunedin 9016, New Zealand townsend.sheena@gmail.com Enriched clone screening Overall, 942 colonies were screened for repeats resulting in 67 (7.1%) positive clones. Of these, 39 were sequenced using an ABI 3730 Genetic Analyser (Applied Biosystems). Sequences were edited and checked for duplicates using SEQUENCHER v3.7 and 21 pairs of primers designed using PRIMER3PLUS (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) (Untergasser et al. 2007) 454 sequencing details Our sequencing run was scaled to 1/8th of a plate on the Roche 454 GS-FLX System (Roche, Penzberg, Germany. This yielded 42,098 fragment reads (7.3Mb sequence overall) with an average read length of 174bp. Reads were screened for microsatellite repeats using MSATCOMMANDER v0.8.1 (Faircloth 2008) to locate di(194), tri- (287) and tetranucleotide (112) repeat regions. Of these, 54 di-, 111 tri- and 33 tetranucleotide repeat-containing reads had sufficient flanking sequence to allow primer design. After eliminating potential duplicates using SEQUENCHER v3.7 we designed 48 primer pairs using PRIMER3PLUS (Untergasser et al. 2007). Location of loci in the zebra finch genome To ensure that microsatellite loci did not represent duplicates, we conducted a BLAST search using NCBI’s BLAST 2 Sequences protocol with the sequences upon which primer development was based included as both reference and searched sequences (Dawson et al. 2006; Olano-Marin et al. 2010). All matches with scores below 50 and E-values less than were ignored. This search indicated no matches between pairs of loci. To determine the zebra finch (Taeniopygia guttata) chromosome locations of loci, we followed the methods of Olano-Marin et al. (2010) see also Dawson et al. (2006). VecScreen (http://www.ncbi.nlm.nih.gov/VecScreen/VecScreen.html UniVec build 6.0) revealed no vector contamination in any sequences. We initially searched clone and 454 sequences using BLAT at the UCSC Genome Bioinformatics website (http://genome.ucsc.edu/) with the Zebra finch Jul. 2008 (WUGSC 3.2.4/taeGut1) assembly. Positions were confirmed using BLASTN 2.0MP-WashU (WUSTL) (http://genome.wustl.edu/tools/blast) and the Taeniopygia guttata–3.2.4chromosomes database with DUST/SEG filter and RepeatMasker. We followed Olano-Marin et al. (2010) in our criteria for non-ambiguous location assignment, accepting matches with an E-value less than . In the case of multiple matches, if the second best match was more than an order of magnitude weaker, we accepted the best match. When the top two matches included the unknown chromosome, we used the named chromosome assignment if the above criteria were met. When these criteria were not met or when BLAT and BLAST did not agree, we searched for sequences using the reference genome at NCBI (http://www.ncbi.nlm.nih.gov/blast). In all cases, except one, this confirmed assignment of the chromosome from the initial BLAST (WUSTL) search (Table S1). We were not able to unambiguously match the locus Pau24 to a homologous position in the zebra finch genome. We further extended our attempt to do so by searching for the Pau24 sequence within zebra finch WGS reads using the NCBI BLAST search. This also failed to return any well-supported, unambiguous matches. Pau8 and Pau26 were assigned unambiguously to chromosomes via both BLAST searches (WUSTL and NCBI) however, BLAT returned ambiguous start positions for these loci. No location was well-supported compared to other potential locations and the top choices did not correspond to confirmed chromosomal matches, therefore we searched NCBI WGS reads and conducted a BLAT search on the top matching homologous zebra finch sequence to find the best supported approximate start locations for these loci. For a third locus, Pau77, the top start position assignment in BLAT was only weakly preferred over other choices, but consistent in terms of chromosome assignment with both BLAST servers. Conducting a BLAT search on the strongest homologous zebra finch sequence for this locus and confirmed the initial location assignment, which was used. Table S1. Supplementary details for 20 South Island robin microsatellite loci. For amplification, loci were combined in multiplex PCR in three groups as indicated. Locus Repeat motif Zebra finch chr Start position Multiplex group Pau1 Pau2 Pau4 Pau6 Pau7 Pau8 Pau9 Pau16 Pau17 Pau24 Pau25 Pau26 Pau28 Pau39 Pau63 Pau66 Pau67 Pau77 Pau81 Pau82 (TG)2 TTTG(TG)12TTTG (AC)19 AG(AC)3 3 2891710 2 5 48006134 3 (AC)6 AA(AC)2 (AAC)2 AC(A)6 1A 54059184 2 (GT)10 1A 16985012 1 (GT)12 5 43897126 2 (TG)13 (TATG)4 4 39599233 1 (TG)22 5 984444 3 (TC)5 . . . (TC)2 TT(TC)3. . . (TC)6 A(AC)12 1A 27557900 1 (AC)7 . . . (TC)5 5 51323773 1 (AC)15 NA NA 2 (TG)13 2 106694637 2 (GTT)12 1 8542288 3 (GT)8 13 16228286 1 (AG)7 5 57668372 1 (GGGA)6 2 39768919 2 (GACA)5 10 7612298 2 (AAAC)5 4 25406454 2 (TTTA)5 1A 54230324 1 (CCT)5 11 12181319 3 (GAG)6 1A 53337441 2 References Dawson DA, Burke T, Hansson B, Pandhal J, Hale MC, Hinten GN, Slate J (2006) A predicted microsatellite map of the passerine genome based on chicken-passerine sequence similarity. Mol Ecol 15:1299–1320. doi: 10.1111/j.1365294X.2006.02803.x Faircloth BC (2008) Msatcommander: detection of microsatellite repeat arrays and automated, locus-specific primer design. Mol Ecol Resour 8:92–94. doi: 10.1111/j.1471-8286.2007.01884.x Olano-Marin J, Dawson DA, Girg A, Hansson B, Ljungqvist M, Kempenaers B, Mueller JC (2010) A genome-wide set of 106 microsatellite markers for the blue tit (Cyanistes caeruleus). Mol Ecol Resour 10:516–532. doi: 10.1111/j.17550998.2009.02777.x Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM (2007) PRIMER3PLUS, an enhanced web interface to PRIMER3. Nucleic Acids Res 35:W71–W74. doi: 10.1093/nar/gkm306