Grade 8 Periodic Table Study Guide
advertisement

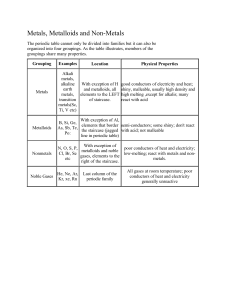
Grade 8 Study Guide The Periodic Table of Elements Test Date: Tuesday, February 25th Definition Term A list of all the known elements The periodic table of elements (PTOE) The PTOE is broken down into 2 groups Metals and non-metals The PTOE is also arranged in vertical columns called Groups or families The PTOE is also arranged in horizontal rows called periods -Extremely reactive -pure in form - silver colored and shiny -1 radioactive element Group 1: Alkali Metals -less reactive than alkali -silver colored -more dense -all solid Group 2: Alkaline Metals The Transition metals: groups 3-12 -mostly solid -good conductors of heat & electricity -1 liquid -few radio active -some synthetic -high densities & melting points -a mix of solids and synthetics -shiny & reactive Lanthanides & Actinides: The Rare Earth Elements Group 13: Boron Group -contains one metalloid and 4 metals - all solids -contains one nonmetal -2 metalloids -2metals Group 14: Carbon Group -all solids -1 gas Group 15: Nitrogen Group Group 16: Oxygen Group -reactive group -1 gas Group 17: Halogens -halogens -very reactive -poor conductors of heat & electricity Group 18: Noble Gases -unreactive nonmetals -colorless -odorless If an element ‘s a.m.u. is in ( ) It is radioactive If the symbol is red, The element is a gas If the symbol is blue It is liquid at room temperature If the symbol is black It is a solid If the element is on the left side of the PTOE It is a metal If the element is on the right side of the staircase effect It is a non-metal If is sits on the staircase effect it is a It is a metalloid If the element is outlined it is Synthetic or man made # of energy levels Period numbers # of valence electrons Group numbers -solid at room temp. -ductile -malleable - excellent conductors of heat and electricity Properties of Metals Separates the metals from the non-metals The staircase effect Any characteristic of matter that can be observed without changing the identity of the material Physical property Amount of mass in a given volume Density Whether a substance is a solid, liquid, or a gas at a particular temperature and pressure State of matter Characteristics of something that allows it to change to something new Chemical property -dull -brittle -low density -low melting point Properties of non-metals properties of Metalloids -brittle -hard -solid at room temperature A substance that conducts electricity only under some conditions semiconductor Elements that have characteristics of both metals and nonmetals Definition of Metalloids The sum of an atom’s protons and neutron Mass number Negatively charged subatomic particle electron