Chapter 9 Science notes
advertisement

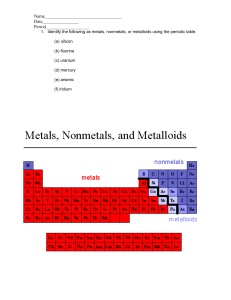
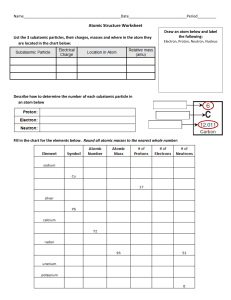
Chapter 9 Comparing Kinds of Matter Lesson 1 Properties of Matter • Mass-the amount of matter in an object; measured on a pan balance; measured in kilograms or grams. • Weight-how strongly gravity pulls on an object; measured in pounds. • Volume-measures how much space matter takes up; a marble’s volume makes the water level rise when you place it in a graduated cylinder. Lesson 1 continued… • Matter-anything that has mass and volume • Density-the amount of mass for each mL (or cm³) of a substance; as you add more marbles to a large box, you are increasing the box’s density Density=mass/volume If a material is dense, matter is packed closely together. Lesson 1 continued… Buoyancy- the resistance to sinking Depends on density If you change the mass or volume of an object, you can change whether or not it will float If you have a toy boat and keep adding mass to it, it will sink Think of a buoy in the water! Lesson 2 Elements • Element-a material that cannot be broken down into anything simpler by chemical reactions. • 3 important properties of elements 1. 2. 3. • • State of matter at room temperature The way they combine with other elements Whether they are metals, nonmetals, or metalloids Most elements are solid at room temperature Metals-elements that share common properties like shiny luster, conductivity, and flexibility Wood is not a good conductor of electricity, but copper is! See the shiny luster! Lesson 2 continued Look at the variety of colors! Each color represents solids, liquids, or gases (metals, non-metals, or metalloids). To See an up close version p. 494 in your textbook has a great example! Lesson 2 continued… • Atom- the smallest unit of an element that keeps the properties of that element. • Nucleus-the hard core/center of an atom; made up of protons and neutrons. Lesson 2 continued… • Proton- a particle with one unit positive charge; the number of protons in an atom is called the atomic number and determines which element it is. • Neutron- a particle with no electric charge; it is neutral • Electron- smaller particle with one unit of negative electric charge each; electrons move around in the space outside the nucleus. Lesson 2 continued… • Molecule- particle with more than one atom joined together. • Elements are grouped together by their properties in the periodic table • On Earth, the most common elements by far are hydrogen and helium Helium Lesson 3 Metals, Nonmetals, and Metalloids • Malleability- the ability to be bent, flattened, or hammered without breaking • Ductility- the ability to be pulled into thin wires without breaking. Gold is both malleable and ductile. Copper is often drawn into wires for conducting electricity. Lesson 3 continued… • Corrosion-when metals combine with nonmetals from the environment. Iron “corrodes” by rusting, which causes the corroded iron to flake away. Lesson 3 continued… • One of the most resourceful metals we use today is aluminum: Often used in mirrors because it is inexpensive and can be polished to be reflective. Aluminum foil wrapped around food will trap heat inside by reflecting it. Can be used to conduct energy cheaply; it is used in electrical wiring, water heaters, and radiators. Easily coated with a thin layer of oxygen to help prevent corrosion.