File
advertisement

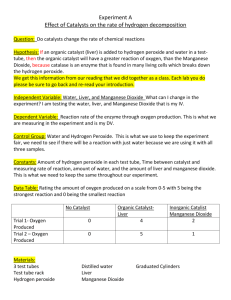
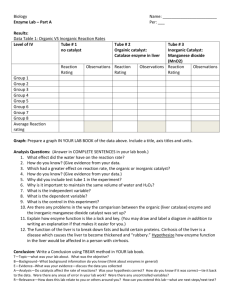
Inorganic and Organic Catalyst Jenna Star Belter jennastar3@gmail.com Biol 1730-517 October 10, 2012 James Smith Wednesday 8-10:50 Abstract Adding a dropper (1 ml) of hydrogen peroxide shows a presence of catalyst enzymes in different substances, if the foam layer becomes present the enzymes are active in the substance. With four different substances, I added 1 dropper (1 ml) of water, and another dropper of hydrogen peroxide to each substance. After I measured the mm of the foam reaction in the test tube, I found the higher the catalyst enzymes in the tested substance, the higher reaction of foam in the test tube. Introduction Conducting the experiment can create a better understanding of substances that may contain catalyst enzymes. The experiment involves filling four individual test tubes with different substances, putting one dropper (1ml) of water in each test tube, then adding one dropper (1 ml) of hydrogen peroxide to each test tube, after one minute report the results of the measured height of the foams. Before testing the experiment, I was looking for action in manganese dioxide and liver extract, showing both an inorganic and organic catalyst reaction. Materials and methods While testing for catalyst in enzymes I took four individual test tubes and filled one test tube one with a spatula of sand, test tube two with a dropper of water (1 ml), test tube three a spatula of manganese dioxide, and test tube four with a drop of liver extract. After each tube was filled with the substance to be tested, I added one dropper (1 ml) of water to each tube, and then one dropper (1ml) with hydrogen peroxide. Next, I waited one minute and measured the thickness of the foam in millimeters, starting from where the water began. Results My results showed manganese and liver extract positively reacted with hydrogen peroxide and broke it down creating foam, while water and sand had no reaction with hydrogen peroxide proving there were no catalyst enzymes in those substances (Fig. 1). The substance with the greatest reaction to hydrogen peroxide was the liver extract, while the smallest reaction was from sand and water, which showed no reaction at all, because of the absence of the hydrogen peroxide (Fig. 2). By my conclusions, I predict a substance will show a reaction to the hydrogen peroxide, depending on how much of a catalyst is in the substance; the higher amount of catalyst, the higher amount of foam. Figure 1 Sample Thickness of Foam Layer (mm) Sand 0 H20 (water) 0 MnO2 (manganese dioxide) 3 Liver extract 31 Figure 2 Foam Layer (mm) Foam Layer vs. Sample 40 30 20 10 0 1 2 3 Test Tube 4 Discussion Presence of hydrogen peroxide will show if a substance contains catalyst enzymes or has an absence of catalyst enzymes. The liver extract contained the highest amount of catalyst, and was the example of an inorganic catalyst. While the manganese peroxide was an organic catalyst, with low but recognizable amount of foam, showing a presence of a catalyst. Depending on which level of the liver extract the same is taken, the amount of catalyst may fluctuate. The results showed that both manganese dioxide and liver extract were two different types of catalyst, but showed action when exposed to hydrogen peroxide. The indication of a foam layer indicated the breakdown of hydrogen peroxide because it shows the oxygen gas being separated by a chemical. Test tubes 1 and 2, filled with the substances sand and water were the controlled substances, if catalyst appears to be in either test tube the experiment was incorrectly done, and needs to be retested. References Pearson. "Enzymes." Symbiosis/Biology. Boston, MA: Pearson Learning Solutions, 2013. 67-69. Print. Reece, Jane B., and Neil A. Campbell. Biology. 9th ed. Boston, MA: Cummings, 2011. Print.