Enzyme Lab: Catalysts & Hydrogen Peroxide Decomposition
advertisement

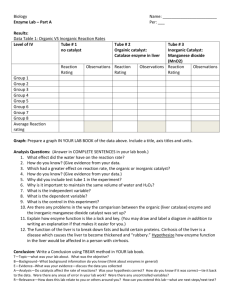
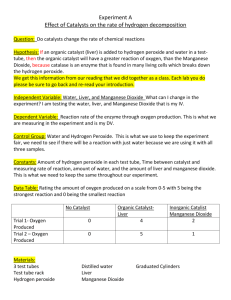
CLASS COPY Do not write on this handout. Biology Enzyme Lab – Part A: The effect of organic and inorganic catalysts on the rate of hydrogen peroxide decomposition. For this lab: You need to include the title, objective, pre-lab questions, hypothesis, summarized procedure, TAPED IN data table, graph, analysis questions, and conclusion. Objective: Measure the effect of catalysts (enzymes) on a chemical reaction in a controlled experiment. Introduction: In each individual cell of a human there are many chemical reactions taking place, performing the necessary functions for being a large, complex, multicellular organism. This is relatively easy to understand. How do these reactions occur? This is not so easy to understand. Chemical reactions involve the breaking and reforming of chemical bonds between molecules (substrate(s) of the reaction), which are transformed into different molecules (product(s) of the reaction). Chemical reactions can occur spontaneously (without added energy or intervention), and indeed many of the chemical reactions necessary for life processes are spontaneous; some however, are not. What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. The enzyme is not altered by the reaction. You have hundreds of different enzymes in each of your cells. Each of these enzymes is responsible for one particular reaction that occurs in the cell. In this lab, you will study an enzyme that is found in the cells of many living tissues. The name of the enzyme is catalase (KAT-uhLAYSE). It speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen. The reaction is: 2 H2O2 + enzyme ----> 2 H2O + O2 This reaction is important to cells because hydrogen peroxide (H2O2) is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells. You will be using chicken or beef liver. It might seem strange to use dead cells to study the function of enzymes. This is possible because when a cell dies, the enzymes remain intact and active for several weeks, as long as the tissue is kept refrigerated. PRELAB Questions: 1. What is an enzyme? 2. What to enzymes do? 3. Define catalyst. 4. Complete the following: All enzymes are _________________, but not all _______________are enzymes. 5. Write the chemical equation above. Circle the reactant(s) in the equation above. Put a square around the product(s) in the equation above. 6. What is our substrate in this lab? 7. What is our enzyme in this lab? 8. What indication will we have that the enzyme is working? HINT: Look at the products, what would having these together LOOK like? Experimental Design Diagram In this experiment you will compare the effect of the type of catalys used on the speed of a reaction. All scientific experiments need to have an independent variable and a dependent variable. Additionally, one must always consider what you will use as a control and what other variables you need to hold constant. In this experiment we will have the following: Independent Variable: Type of catalyst added Level of IV No catalyst Organic catalyst: Inorganic Catalyst: Catalase enzyme in liver Manganese dioxide (MnO2) Number of 1 per group 1 per group 1 per group repeated trials for each level Dependent Variable: Speed of Reaction Throughout this investigation you will estimate the rate of the reaction (how rapidly the solution bubbles) on a scale of 0-5 (0=no reaction, 1=slow, ..... 5= very fast). Control: (What you compare your results to) Test tube # 1 with no catalyst added. Constants: (Factors you kept the same because they could affect the rate of reaction) - Amount of hydrogen peroxide in each test tube - Time between adding catalyst and measuring rate of reaction - Are there others we ought to consider? Materials: 3 test tubes 1 test tube rack Hydrogen peroxide (H2O2) Distilled water Liver Manganese dioxide(MnO2) Graduated cylinder(s) Procedure: 1. SAFETY: Wear goggles throughout the lab. 2. Obtain three test tubes and label them 1, 2, and 3. 3. VERY IMPORTANT: DO NOT ADD THE H2O2 to tubes #2 and 3 until you are running the experiment for that test tube. 4. Prepare the contents of each test tube as outlined below ONE AT A TIME: Test tube #1 – 10 mL Water and 10 mL hydrogen peroxide (H2O2) Test tube #2 – 10 mL Water and 10 mL H2O2 and small piece of liver Test tube #3 – 10 mL Water and 10 mL H2O2 and small (pea size) amount of MnO2 5. Record your observations about each tube in addition to rating the reaction (how rapidly the solution bubbles - on a scale of 0-5 where 0=no reaction, 1=slow, ..... 5= very fast) 6. Clean up as instructed. 7. Record the data from the other classroom groups. 8. Calculate the average the reaction ratings for each test tube (sum the ratings and divide by the number of entries). 9. Create a graph of your results. 10. Answer the analysis questions.