Chemistry Class Notes: Hydrogen Peroxide & Thermite Reactions
advertisement
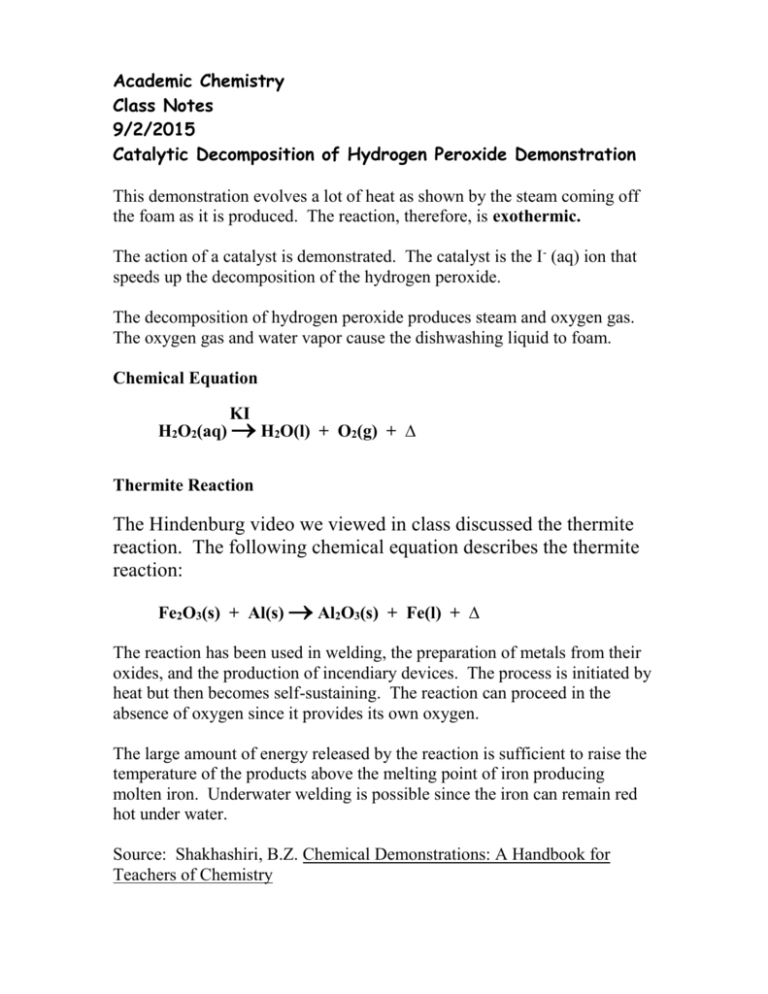
Academic Chemistry Class Notes 9/2/2015 Catalytic Decomposition of Hydrogen Peroxide Demonstration This demonstration evolves a lot of heat as shown by the steam coming off the foam as it is produced. The reaction, therefore, is exothermic. The action of a catalyst is demonstrated. The catalyst is the I- (aq) ion that speeds up the decomposition of the hydrogen peroxide. The decomposition of hydrogen peroxide produces steam and oxygen gas. The oxygen gas and water vapor cause the dishwashing liquid to foam. Chemical Equation KI H2O2(aq) H2O(l) + O2(g) + ∆ Thermite Reaction The Hindenburg video we viewed in class discussed the thermite reaction. The following chemical equation describes the thermite reaction: Fe2O3(s) + Al(s) Al2O3(s) + Fe(l) + ∆ The reaction has been used in welding, the preparation of metals from their oxides, and the production of incendiary devices. The process is initiated by heat but then becomes self-sustaining. The reaction can proceed in the absence of oxygen since it provides its own oxygen. The large amount of energy released by the reaction is sufficient to raise the temperature of the products above the melting point of iron producing molten iron. Underwater welding is possible since the iron can remain red hot under water. Source: Shakhashiri, B.Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry











