Lab 1
advertisement

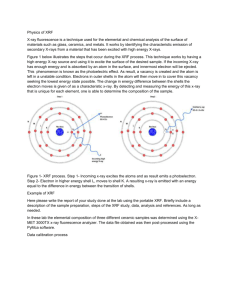
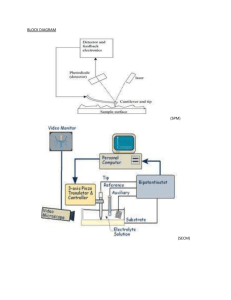
Lab #1: Becoming and Expert-SPM and XRF Purpose: The purpose of this lab is to work with the XRF for one week and then the SPM for one week to essentially become an expert on these two instruments. With the XRF the objective is to obtain data of a standard as well as an unknown. The SPM objective is to obtain images at 4.75µm and 1.0µm scan sizes of the calibration gradient then take a 5µm image of the CD sample. Introduction: X-Ray Fluorescence (XRF) is a powerful tool for rapid quantitative determinations used for routine, relatively non-destructive chemical analysis of rocks, minerals, sediments, and fluids. This technique bombards the sample with X-rays exciting the material. When materials are excited with highenergy, short wavelength radiation, they become ionized. This causes energy to be released due to the decreased binding energy causing the emitted radiation to be of lower energy then the primary incident x-rays. This is termed fluorescence radiation. Scanning Probe Microscopy (SPM) is a branch of microscopy that forms images of surfaces using a physical probe that scans the specimen. It is the primary tool used to image at the nano level. An image of the surface is obtained by mechanically moving the probe in a raster scan of the specimen and recording the probe-surface interaction as a function of position. SPM covers several related technologies for imaging and measuring surfaces on a fine scale. Various interactions can be studied depending on the mechanics of the probe. Week 1: Day 1: 1. Learned how to add liquid nitrogen to the XRF instrument. 2. Learned how to turn on the XRF as well as run the computer program that goes along with it. 3. Ran a standard and obtained accurate results for CuKa. Day 2: 1. Added liquid nitrogen to the XRF. 2. Ran a standard and obtained accurate results for CuKa values. 3. Ran an unknown and compared it to the standard. Week 2: Day 1: 1. Learned how to turn the SPM on and the computer programs that go along with it. 2. Was able to go through all the steps of aligning the laser. 3. Had some troubleshooting and was not able to take an image. Day 2: 1. Was able to run the instrument with less trouble shooting this time. 2. Was able to obtain an image of the calibration gradient at 4.75 µm and 1.0 µm. 3. Was able to also obtain an image of the CD sample at 5 µm. XRF Procedure: Follow SOP placed on my website. Important to know for lab: XRF requires liquid nitrogen to be placed in the instrument during use after every approximately 8 hours, so do not over-looked this step on the SOP Even if there is liquid nitrogen in the instrument, it is good just to top it off before using the instrument and the liquid nitrogen levels running to low Without liquid nitrogen the x-ray will overheat! SOP very straight forward, so just follow it word for word SPM Procedure: Follow SOP placed on my website. Important to know for lab: Make sure tip has been placed on correctly Click approach instead of manually clicking the Z bar downwards because you will not be close enough in the end to take an image o If the red line does not appear on the graph on the imaging mode this is most likely why When on imaging mode, we made the red and green lines become horizontal just by clicking the arrows next to the A but you can do this by changing the slope as well Data: XRF Data Results: SPM Data Results: 4.75 µm calibration gradient in 3D: 1µm calibration gradient in 3D: 4.75 µm CD sample in 3D 5µm image: Calculations: No calculations were necessary for either of these instruments. Conclusions: The XRF we did not have any difficulties with and found it quite easy to use. We were able to identify that the standard and unknown were made up of different materials after collecting data on each of them. The A750 standard was ~93% Aluminum while the unknown was ~91% Silicon. With both the unknown and the standard CuKa it was 8.04± 0.04 KeV leading us to believe that both materials were similar amongst their peak list in that area. Overall, the XRF was not complicated to use and we did not have any trouble-shooting while operating this instrument. The SPM was a little more difficult for us to operate, and we had more trouble-shooting with this instrument. It was overall more difficult to use then the XRF. We still were able to obtain images of the calibration gradient at 4.75µm and 1.0 µm besides all the issues we had with this instrument. We were also able to obtain an image of the CD sample using a 5 µm image size. From the images obtain we were able to see a distinct difference between the two different samples.