Lab 3: SPM, XRF
advertisement

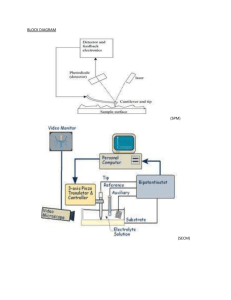
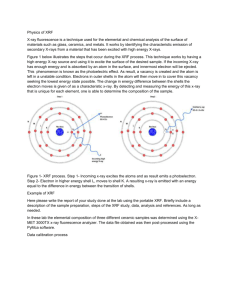
SPM/XRF Lab Report Introduction: SPM stands for Scanning Probe Microscopy. It is a type of microscopy that forms images of a surface using an actual probe and moving it around the samples surface. The microscope records the probe-surface interaction as a function of the samples position. The microscope and probe have a very good atomic resolution and samples can be observed in air at standard temperature and pressure or when dissolved in liquids. However the instrument does take a while to obtain an image. Most of the time spent with the SPM is trying to center the laser on the probe and being very careful not to break the probe while scanning the sample, also the images are generally smaller because they are on a nanometer scale and can sometimes be hard to see. XRF stands for X-Ray Fluorescence and it is a form of spectroscopy that emits characteristics of X-rays from a material that has been excited by hitting it with x-rays or gamma rays. The XRF that is used in the lab has x-rays, they are safer to use and are inexpensive to the other instruments. It is mainly used for metal concentrations in samples. It will give an analysis about how much of certain elements are in a metal sample. The XRF is very good with bulk analysis of major and trace elements but not with distinguishing between isotopes or ions of the same element. Purpose: The purpose of these experiments was to get acquainted with the SPM and XRF, one day was spent working with the SPM and the other day was with the XRF. For the SPM we wanted to figure out how to scan and probe a sample properly and obtaining an image. The XRF was used to determine the element content of a known and unknown metal sample. Methods: Day 1: For the first day we spent the time working on the SPM. We figured out how to operate the instrument and load the samples and work the software. While trying to center the laser and probe we found one tip broken and then accidently broke another tip and the lab assistants did not come to instruct us on how to put new tips in. After figuring out how to do it by ourselves we spent majority of the rest of the time trying to center the laser with the probe but were not successful. Day 2: After having an unsuccessful day on the SPM, the XRF was much easier to figure out and operate. We loaded the instrument with liquid nitrogen and waited 30 minutes to allow it to cool down and for the x-rays to work. Then we started the computer and software and waited an additional 30 minutes for the software to warm up. When everything was ready to go, we calibrated the XRF with the known sample of A750. When the calibration was complete we ran the A750 again for our known sample and then used the SUS sample for the unknown and compared the element contents of both for the analysis. Results: The results for both the known and unknown samples can be found in the lab notebook. We did not receive any results for the SPM. We could not get the laser to center with the probe tip, therefore we were not able to probe sample for calibration of the instrument and to run a CD sample to have for comparison. If everything went as planned we should have gotten scans at different sizes at 1um, 4.75 um, and 5 um and then obtain an image for all scan sizes. We should have been able to compare the image size differences based on the scan sizes. Also we should have been able to at least get a CD or DVD sample done at a certain scan size to identify the structural characteristics at a 5um scan size. As for the XRF we were able to obtain results from both the known and unknown sample. For the known we had to compare the KeV of CuKa to that of a known value of CuKa. The actual value of CuKa was 8.04 KeVand we were able to get a value of 8.04 KeV as well, which tells us that our sample has the same amount of CuKa being used when compared to that of a standard value. Also the known sample was 93.14% Aluminum along with a small amount of other trace elements in the sample. This sample had lighter density and had a dull tint to it. The unknown sample came out to have a 7.98 KeV meaning that it had a little less voltage and current than the known sample. Also it was 71.24% Iron and 19.34% Chrominum along with some other trace elements. Also the unknown sample had more shine than the known and was heavier. Just from handling the two samples we could tell that the unknown was heavier and most likely had more lead than the other. Conclusion: Overall this week was a learning week. After all the problems with the SPM we were able to come back from that and have a better day during the second lab. When we didn’t get any help for trying to figure out the SPM, we were able to figure out how to do things on our own, but that took up time that we didn’t have. We could have used that time to run samples and actually obtain results from the day as opposed to struggling with the instrument the entire time. Instead of getting frustrated with not being able to finish on time or to get anything done for that matter, we could have finished at least the calibration and one sample if someone came to help when we asked for a little assistance. The XRF was very successful, the instructions were easy to follow and when we asked questions they were answered. The instrument was fast and efficient and we did not run into any problems with running the instrument, loading samples, or actually figuring out how the instrument works. The instrument was one of fast pace and we were able to finish early and obtain excellent results that backed up what is known about the samples and the standards for the XRF. Just by sight we were able to distinguish that there was a difference between the two samples and when run through the XRF, we got actual results that the samples were different and contained very different elemental properties. Having success on this instrument ended a frustrating lab week on a good note and gave a boost into the next lab week.