XRF Lab
advertisement

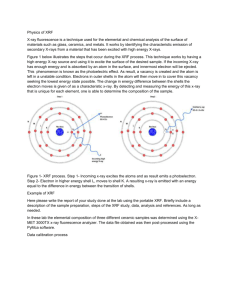
XRF Lab Introduction: XRF stands for x-ray fluorescence. The x-ray fluorescence works by bombarding a sample with x-rays, which inject inner core electrons. As a result, electrons fill energy holes. Energy is then released in the form of fluorescence, which can be used to identify a variety of components of a sample. The purpose of this lab is to become familiar with the XRF and to run standards as well as a meteorite sample. Method: 1) Go to the gas room and fill a large dewer with liquid nitrogen. Record how much liquid nitrogen is taken. 2) Transfer the liquid nitrogen to the 1 liter dewer. 3) Open the detector cover and remove the center piece. 4) Fill the detector to about mid-way up the column with liquid nitrogen (how much liquid nitrogen is used is dependent on whether or not there is already some in the XRF) 5) Put the center piece back in and close the cover. After closing the cover let the XRF sit for about thirty minutes. 6) Power on the XRF and let it stabilize for fifteen minutes 7) After the XRF has stabilized, click on the PCEDX icon and click ok on the pop up window that is asking to stabilize the instruments 8) Another pop up window will appear. Click ok. This will turn on the x-ray. 9) Let the XRF sit for another thirty minutes while the x-ray is warming up. 10) Calibrate the XRF by closing the analysis window, clicking the maintenance icon, and then clicking instrument calibration. 11) Locate the standard in the drawer below the XRF. If the XRF is not already open, click open on the front of the instrument. 12) Place the standard sample over the hole on the back of the XRF. 13) Click yes and then start in the calibration window now open on the computer. The door to the XRF will close automatically. 14) After the calibration is complete, close the instrument calibration and maintenance windows. 15) To analyze the standard, click on the analysis icon and then click analytical group. 16) In the drop down box next to Owner of Analytical Group, select System Group. Then select Easy and click ok. 17) Click start in the analysis window. If the XRF does not close automatically, push and hold close on the front of the XRF. 18) After the analysis is complete, a result window will appear. Check to make sure that the Cu-Ka transition is at an energy of 8.04 +/- 0.004 KeV. This will be under Peak List. 19) Remove the standard and place it back in the drawer. Remove the sample from the drawer. 20) Place the meteorite sample over the hole in the back of the XRF. 21) Follow steps 15-16. 22) To shut down the instrument, first close the analysis window, then click the maintenance icon. 23) Click instrument set up, select x-ray power off, and then click execute shut down. 24) If the instrument is to be fully shut down, wait thirty minutes after turning off the x-ray and then press the off button on the instrument. Data: The data obtained from using this instrument includes a quantitative result. The quantitative result shows the percentage of an analyte in the sample. Print outs of the quantitative results are in my notebook. XRF Standard Data Analyte Al Sn Si Cu Ni Fe Ca Ta Ti Zn Cr Mn Se XRF Meteorite Sample Data Result 92.644% 3.017% 2.918% 0.771% 0.225% 0.201% 0.094% 0.038% 0.032% 0.020% 0.019% 0.017% 0.005% Analyte Mn Si Fe Al Ca Ti Cr Ni Cu Result 63.139% 17.221% 14.535% 4.481% 0.230% 0.163% 0.123% 0.071% 0.039% Conclusion: Overall, the XRF was a very simple instrument to use. I actually enjoyed using the XRF and I am interested in learning more about what the instrument can be used for. The standard sample analyzed contained mostly aluminum, with small amounts of tin and silicon, as well as trace amounts of copper, nickel, iron, calcium, zinc, and a few other elements. The meteorite sample contained mostly manganese, silicon, and iron. The meteorite sample also contained a small amount of aluminum, as well as trace amounts of calcium, nickel, copper, chromium, and titanium. What a meteorite is made of depends on where the meteorite comes from.