X-Ray Fluorescence Spectrometry
advertisement

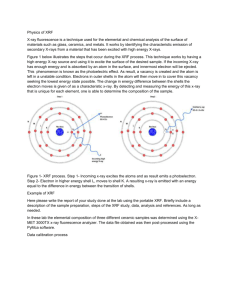
X-Ray Fluorescence Spectrometry Krista Amato and Jennifer Hernandez Introduction The purpose of this experiment was to analyze a specimen of ceramic pottery from the Pueblo Indians using an X-MET 3000TX XRF instrument. Only the back of this specimen was analyzed, designated by 17153-A. Physics of XRF X-Ray fluorescence (XRF) is a non-destructive technique used for elemental analysis and chemical analysis of minerals, rock, sediments, etc. XRF is a three step process. First, ionization takes place in which an atomic electron is hit by an external X-ray. A core hole is left due to the ejection of the atomic electron. An atomic electron from a higher energy level fills the core hole in which it releases its energy as an X-ray photon. The electrons can be emitted from different levels and Auger electrons can be emitted during this process. The energy is measured due to the capturing of X-rays which is used to identify the elements that are emitted. XRF spectrometers work either by wavelength-dispersive principles or energy-dispersive principles. Wavelength-dispersive analysis separates the wavelengths of the radiation, which is similar to an electron microprobe. However, the spot size isn’t as small as an electron microprobe, so this technique is more commonly used for bulk analysis of larger materials. Energy-dispersive analysis sorts the energies of the photons. Once analyzed, the intensity of the characteristic radiations is directly related to the amount of each element in the material. When an atomic electron is removed by an energetic photon, an electron from a higher energy fills the hole. This process is denoted by certain names. For instance, an electron that fell from the L shell to the K shell is usually denoted as Kα, MK transition as Kβ, and ML as Lα. A fluorescent photon with a characteristic energy is yielded due to these transitions which is equal to the difference of the initial orbital and final orbital. Planck’s law is used to determine the wavelength of the fluorescent photon: λ = h ∙ c / E. Fig. 1 shows the process of X-ray fluorescence. Experimental Details The XRF was placed on a stand facing upwards. The piece of pottery (as shown in Fig. 2) was carefully clamped with bubble wrap onto a stand. The pottery sample was placed directly over the XRF and lowered until it contacted the detector. The XRF was then turned on, and the test was run for 300 seconds. The sample was moved and tested twice more in different areas. Fig. 2 shows the pottery specimen tested using X-ray fluorescence. Results The data was calibrated by fitting the Ag peak generated by the XRF machine directly. The highest peaks were then fitted to identify the compostion of each respective peak. Below is the spectra and the mass fraction for each element identified. 1. MASE_17153A-1-back Element K Ca Ti Cr Mn Fe Ni Cu Zn Ga As Rb Sr Y Zr Nb Ag W Au Group K K K K K K K K K K K K K K K K K L L Fit Area 1.76E+03 2.25E+03 1.94E+03 4.22E+02 6.09E+02 9.10E+04 1.42E+02 3.53E+02 2.04E+03 1.18E+02 3.89E+02 3.12E+03 1.85E+04 2.88E+03 1.14E+04 2.30E+03 8.42E+03 2.39E+02 7.32E+03 Sigma Area 5.03E+01 5.70E+01 5.16E+01 3.12E+01 3.80E+01 5.30E+02 2.18E+01 2.77E+01 5.60E+01 2.97E+01 4.78E+01 1.18E+02 2.03E+02 1.23E+02 1.85E+02 1.30E+02 1.87E+02 6.62E+01 1.33E+02 Mass fraction 0.004433 0.001153 0.0001208 6.33E-06 5.44E-06 0.0005077 3.80E-07 7.45E-07 3.33E-06 1.59E-07 3.60E-07 1.64E-06 8.61E-06 1.19E-06 4.26E-06 7.73E-07 1.78E-06 3.68E-07 7.09E-06 2. MASE_17153A-2-back Element K Ca Ti Cr Mn Fe Ni Cu Zn Ga As Rb Sr Y Zr Nb Ag W Au Group K K K K K K K K K K K K K K K K K L L Fit Area 2.32E+03 2.95E+03 2.49E+03 4.87E+02 9.02E+02 1.11E+05 1.45E+02 3.13E+02 2.10E+03 1.73E+02 3.93E+02 3.72E+03 1.91E+04 2.46E+03 1.30E+04 1.84E+03 8.81E+03 1.90E+02 7.38E+03 Sigma Area 6.29E+01 7.28E+01 6.27E+01 3.22E+01 4.19E+01 1.35E+03 2.05E+01 2.59E+01 5.84E+01 2.78E+01 4.42E+01 1.17E+02 2.57E+02 1.14E+02 2.13E+02 1.19E+02 1.87E+02 6.05E+01 1.46E+02 Mass fraction 0.005822 0.001515 0.0001555 7.31E-06 8.06E-06 0.0006192 3.90E-07 6.60E-07 3.42E-06 2.33E-07 3.64E-07 1.96E-06 8.89E-06 1.02E-06 4.86E-06 6.18E-07 1.86E-06 2.93E-07 7.14E-06 3. MASE_17153A-3-back Element K Ca Ti Cr Mn Fe Ni Cu Zn Ga As Rb Sr Y Zr Nb Ag W Au Group K K K K K K K K K K K K K K K K K L L Fit Area 2.11E+03 2.73E+03 2.15E+03 1.56E+02 7.92E+02 1.15E+05 1.37E+02 4.46E+02 2.13E+03 1.58E+02 3.17E+02 3.23E+03 2.19E+04 2.74E+03 1.40E+04 2.73E+03 1.14E+04 3.78E+02 8.14E+03 Sigma Area 5.53E+01 6.58E+01 6.32E+01 3.95E+01 5.76E+01 3.44E+02 2.25E+01 3.01E+01 5.70E+01 3.09E+01 4.95E+01 1.26E+02 1.97E+02 1.30E+02 1.90E+02 1.39E+02 1.93E+02 6.62E+01 1.34E+02 Mass fraction 0.005301 0.001403 0.000134 2.34E-06 7.07E-06 0.0006423 3.68E-07 9.40E-07 3.47E-06 2.12E-07 2.94E-07 1.70E-06 1.02E-05 1.13E-06 5.22E-06 9.16E-07 2.42E-06 5.82E-07 7.88E-06 4. MASE_17153A Average Mass Fraction Values Element K Ca Ti Cr Mn Fe Ni Cu Zn Ga As Rb Sr Y Zr Nb Ag W Au Mass fraction .005185 .001357 .0001368 5.323E-06 6.856E-06 5.897E-04 3.793E-07 7.818E-07 3.405E-06 2.014E-07 3.391E-07 1.767E-06 9.231E-06 1.111E-06 4.779E-06 7.691E-07 2.020E-06 4.142E-07 7.370E-06 Conclusion Since multiple readings were taken from the same sample, the mass fractions were very similar as well as the spectra.