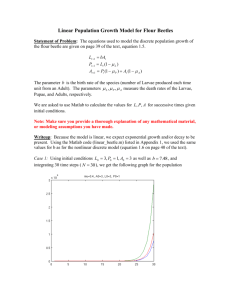
Supplemental 3. I. Determination of the enantiomeric ratios of
advertisement

Supplemental 3. I. Determination of the enantiomeric ratios of pheromone components of D. frontalis and D. mesoamericanus. A) Frontalin and endo-Brevicomin. Volatiles collected from individual females of either species that had fed singly in a log of Pinus oocarpa for 1 d were analyzed by enantioselective gas chromatography. The samples were those for which non-enantiomeric pheromone production data had been published previously (Sullivan et al., 2012). These insects had been collected within the same general area (<15 km distance) as the insects used in the experiments of the present study. The methods for sample collection are described on page 820 of Sullivan et al. (2012); the results of the non-enantiomeric, quantitative analysis of detected pheromone components in these samples are presented in Table 2 under the heading “Solitary in Log 1 d”. Nine samples from D. mesoamericanus and 10 from D. frontalis were analyzed in splitless mode on a Hewlett-Packard GC-MS (Model 6890-5973) fitted with a Supelco (Bellefonte, Pennsylvania, United States of America) gamma-Dex 225 column (30 m length x 0.25 mm i.d. x 0.25 µm film thickness) which provided excellent separation of the enantiomers of frontalin and endo-brevicomin. The oven program was 40° for 1 min, then 5°/min to 220°, then held 7 min. Enantiomers were identified by matching mass spectra and retention times to those of enantiomeric standards provided by Contech (previously PheroTech; Victoria, British Columbia, Canada). Enantiomeric ratios (as percentage of the more abundant enantiomer) were determined from the ratio of peak integration areas of single ion chromatograms of diagnostic ions for each compound (for frontalin m/z 100; for endo-brevicomin m/z 114). Ratios were not corrected with a dilution curve of known quantities of the enantiomers of the target compounds, however previous analyses with the same instrument indicated that the ratio of mass to integration area was approximately constant within the range of concentrations analyzed. (-)-Frontalin was the dominant enantiomer for both D. mesoamericanus and D. frontalis females [D. mesoamericanus, 95.0% (±0.5% S.D.); D. frontalis, 94.3% (±0.9% S.D.)] whereas D. mesoamericanus females produced >99% (+)-endo-brevicomin. Dendroctonus frontalis males sampled within the southeastern United States of America also produce apparently >99% (+)-endo-brevicomin (Sullivan et al. 2007). B) Ipsdienol. Pooled subsamples of the volatiles collections of either D. mesoamericanus gallery entrances (this study) or frass collected from the same females (manuscript in preparation) were concentrated an additional 10-fold. These two pooled samples were then analyzed on a HewlettPackard model G1800C GC-MS fitted with a Supelco beta-Dex 120 capillary column (30 m length x 0.25 mm i.d. x 0.25 µm film thickness) which permitted good resolution of the enantiomers of ipsdienol. The oven program was 50° for 1 min, then 4°/min to 220° and held 10 min. Enantiomeric ratios were calculated from the single ion chromatogram of m/z 85. The pooled frass aeration samples of female D. mesoamericanus contained approximately 98% (+)ipsdienol. The pooled entrance aeration samples of female D. mesoamericanus possessed a 3:97 ratio of the 85 m/z peak areas at the retention times of (-) and (+) ipsdienol, respectively; however an apparent coeluent at the retention time of (-) may have inflated the peak area of this enantiomer slightly. Consistent with these results, additional analyses of four samples of female D. mesoamericanus mining in logs (samples reported in Sullivan et al. 2012) indicated that these insects produced >95% (+)-ipsdienol. II. Implications of use of racemic alpha-pinene, frontalin, and ipsdienol in assayed synthetic blends: (1) alpha-Pinene: The enantiomeric ratio of the alpha-pinene produced by the P. oocarpa logs of the current study was not determined, but pines produce both (+) and (-)-alpha-pinene in varying ratios (Klimetzek and Francke 1980, Marques et al. 2012). The racemic mixture seemed to be a reasonable “default”, and use of the racemate was likely inconsequential to the experiments since the alpha-pinene component was presented uniformly in all treatments. (2) Frontalin: Availability determined our use of the racemate. Research on the behavioral effects of the enantiomers of frontalin in D. frontalis has indicated that there are no antagonistic affects between enantiomers and the (-)-enantiomer appears to be more attractive than the (+)enantiomer (Payne et al. 1982). In walking olfactometer assays, responses to a racemic mixture did not differ significantly from responses to the (-)-enantiomer. When frontalin has been shown to be an attractant for Dendroctonus (Wood et al. 1976, Phillips et al. 1990, Lindgren 1992), the enantiomers have not been found to be antagonistic although the (-)-appears to be the more active enantiomer. (3) Ipsdienol: Availability determined our use of the racemate. It is well-known that enantiomeric ratio of ipsdienol can be highly specific and enantiomers can be antagonistic in Ips DeGeer (Seybold 1993). Few Dendroctonus spp. have been found to produce ipsdienol (Skillen et al. 1997), however a study on the effect of ipsdienol enantiomers on D. ponderosae Hopkins [which like D. mesoamericanus produce the (+)-enantiomer)] indicated inhibitory effects on response to attractants with generally no difference in activity between the (+)-enantiomer and the racemate (Hunt and Borden 1988). In trapping experiments in Chiapas, (+)-ipsdienol inhibited attraction by D. frontalis males whereas the (-)-enantiomer did not alter beetle responses (authors’ unpublished data). Hunt DWA, Borden JH (1988). Response of mountain pine beetle, Dendroctonus ponderosae Hopkins, and pine engraver, Ips pini (Say), to ipsdienol in southwestern British Columbia. J Chem Ecol 14: 277-293. Klimetzek D, Francke W (1980) Relationship between the enantiomeric composition of α-pinene in host trees and the production of verbenols in Ips species. Experientia 36: 1343-1345. Lindgren BS (1992) Attraction of Douglas-fir beetle, spruce beetle and a bark beetle predator (Coleoptera: Scolytidae and Cleridae) to enantiomers of frontalin. J Entomol Soc B C 89:13-17. Marques FA, Frensch G, Zaleski SRM, Nagata N, Sales Maia BHLN, Lazzari SMN., Lenz CA, Corrêa AG (2012) Differentation of five pine species cultivated in Brazil based on chemometric analysis of their volatiles identified by gas chromagraphy-mass spectrometry. J Braz Chem Soc 23:1756-1761. Payne, TL, Richerson JV, Dickens JC, West JR, Mori K, Berisford CW, Hedden RL, Vité JP, Blum M S (1982) Southern pine beetle: Olfactory receptor and behavior discrimination of enantiomers of the attractant pheromone frontalin. J Chem Ecol 8: 873-881. Phillips TW, Nation JL, Wilkinson RC, Foltz JL, Pierce HD, Jr., Oehlschlager AC (1990) Response specificity of Dendroctonus terebrans (Coleoptera: Scolytidae) to enantiomers of its sex pheromones. Ann Entomol Soc Am 83: 251-257. Seybold SJ (1993) Role of chirality in olfactory-directed behavior - Aggregation of pine engraver beetles in the genus Ips (Coleoptera, Scolytidae). J Chem. Ecol 19: 1809-1831. Sullivan BT, Shepherd WP, Pureswaran DS, Tashiro T, Mori K (2007) Evidence that (+)-endobrevicomin is a male-produced component of the southern pine beetle aggregation pheromone. J Chem Ecol 33:1510-1527 Sullivan BT, Niño A, Moreno B, Brownie C, Macías-Sámano J, Clarke SR, Kirkendall LK, Zúñiga G (2012) Biochemical evidence that Dendroctonus frontalis consists of two sibling species in Belize and Chiapas, Mexico. Ann Entomol Soc Am 105:817-831 Skillen EL, Berisford CW, Camaan MA, Reardon RC (1997) Semiochemicals of forest and shade tree insects in North America and management applications. USDA Forest Service Forest Health Technology Enterprise Team Publication FHTET-96-15. Wood DL, Browne LE, Ewing B., Lindahl K, Bedard WD, Tilden PE, Mori K, Pitman GB, Hughes PR (1976) Western pine beetle - Specificity among enantiomers of male and female components of an attractant pheromone. Science 192:896-898.