Chapter 5- Chirality
advertisement

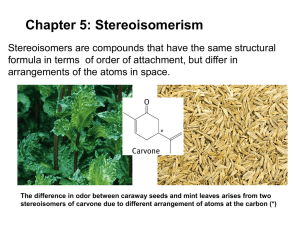
Chapter 5- Chirality Chirality • A chiral object is an object that possesses the property of handedness • A chiral object, such as each of our hands, is one that cannot be placed on its mirror image so that all parts coincide • Another words, a chiral object is not superposable on its mirror image • Ex. Isomer Review • Isomers are different compounds that have the same molecular formula • Constitutional Isomers have the same molecular formula but different connectivity • Stereoisomers are not constitutional isomers • Stereoisomers have the same connectivity but different arrangement of atoms in space Isomer Review • We have seen some forms of stereoisomers: – Cis/Trans isomers in alkenes – Cis/Trans forms of substituted cyclic molecules • Stereoisomers can be subdivided into two general categories: enantiomers and diastereomers New Definitions • Enantiomers- are stereoisomers whose molecules are nonsuperposable mirror images of each other • Diastereomers- are stereoisomers whose molecules are not mirror images of each other – Cis/tran isomers are one type of diastereomer Chirality • Enantiomers occur only with compounds whose molecules are chiral • Chiral Molecule- is defined as one that is not superposable on its mirror image • Alkene stereoisomers are not chiral Chirality • Examples of Chiral Molecules • A chiral molecule and its mirror image are called a pair of enantiomers. • Molecules that are superposable on their mirror image are achiral. Example of a Chiral Molecule • Consider 2-butanol • Until now, we have presented the formula as one compound • But there are actually two different molecules of 2-butanol • They are enantiomers! When to expect enantiomers • How do we know when to expect the possibility of enantiomers? • One way, but not the only way, is to recognize that a pair of enantiomers is always possible for molecules that contain one tetrahedral atom with four different groups attached to it • For 2-butanol, it is C2 Stereogenic Carbon • In enantiomers, exchanging any two groups at the chiral carbon converts one enantiomer into the other. • Stereogenic Carbon- a carbon atom bearing groups of such nature that an interchange of any two groups will produce a stereoisomer. • Enantiomers do not interconvert since this would require breaking covalent bonds. Achiral Compounds • If all the tetrahedral atoms in a molecule have two or more groups attached that are the same, the molecule does not have a stereogenic carbon • The molecule is superposable on its mirror image and is achiral. • Read section 5.5, page 199, about the Biological importance of Chirality Testing for Chirality • The ultimate way to test for molecular chirality is to construct models of the molecule and its mirror image and then determine whether they are superposable. • If they are superposable, the molecule is achiral. • If they are not superposable, then the molecule is chiral. Other Aids • There are other aids to help recognize chiral molecules 1) The presence of a single tetrahedral stereogenic carbon. (If there is more than one, it may or may not be chiral!) 2) Planes of symmetry- if a molecule posses a plane of symmetry, it will not be chiral Plane of Symmetry • Plane of Symmetry- an imaginary plane that bisects a molecule in such a way that the two halves of the molecule are mirror images of each other. • The plane may pass through atoms, between atoms, or both. • Ex. • All molecules with a plane of symmetry are achiral. Nomenclature of Enantiomers: The R/S System • Uses the Cahn-Ingold-Prelog system • Rules: 1) Assign each group attached to the stereogenic center a priority. Priority is assigned based on the atomic number of the atom directly connected to the stereogenic center. In case of isotopes, the greater atomic mass has higher priority Nomenclature of Enantiomers: The R/S System 2) When priority cannot be assigned based on the atom directly attached, move to the next set of atoms until a point of difference is found. 3) Rotate the molecule so that the group with the lowest priority is pointing away from you. Then trace a path from 1 to 2 to 3. Nomenclature of Enantiomers: The R/S System • If the path is clockwise, the enantiomer is designated (R) • If the path is counter clockwise, the enantiomer is designated (S) 4) Groups containing double or triple bonds are assigned priorities as if both atoms were duplicated or triplicated. Enantiomer or Same Molecule? • Deciding whether molecules are enantiomers or the same molecule can be challenging! • There are sever options available for this task: 1) Build model and check for superposability 2) Rotate molecules on paper 3) Exchange groups (remember, exchanging two groups changes configuration) 4) Name the molecule Properties of Enantiomers • Enantiomers are not like constitutional isomers • Enantiomers have many of the same chemical and physical properties, such as MP, BP, solubility, density, etc • Enantiomers only show different behavior when the interact with other chiral substance. Properties of Enantiomers • The easiest observable difference in enantiomers is their behavior towards planepolarized light. • When a beam of plane-polarized light passes through an enantiomer, the plane of polarization rotates • Each enantiomer rotates the beam an equal amount, but in opposite directions Interaction with Plane-Polarized Light • Because of these interactions, separate enantiomers are said to be optically active compounds. • These interactions are measured with a polarimeter Plane-Polarized Light • Normal light exist with 2 perpendicular oscillating fields, an electric field and a magnetic field • We are only concerned with the electric field • If we looked down a normal beam of light, we would see many different electric fields in every possible plane • If the light beam passes through a polarizer, the light that emerges would only be in one plane. • That is called plane-polarized light. Polarimeter • Clockwise rotation (+) dextrorotatory • Counterclockwise (-) levorotatory • This designations have nothing to do with R/S configurations! Specific Rotation • The number of degrees the plane rotates depends on the number of chiral molecules it passes through • In order to place measured rotations on a standard basis, we calculate a quantity called specific rotation, [α] α α = 𝑐 ×𝑙 α =specific rotation α=observed rotation c= concentration (g/mL) l= length of smaple (dm) Specific Rotation • When reported, you will see a superscript and subscript on the right side of the [α]. • Superscript is the temperature • Subscript indicates the type of light used. Racemic Forms • Consider 2-butanol • We saw earlier there are two forms: – A R form and a S form • Each time plane polarized light passes through the R form, the plane rotates a little to the left • If the sample contains some S form, the plane will shift back to the right as it passes them. Racemic Forms • If there are equal amounts of R and S, then there will be no net rotation of the plane • This equal mixture of two enantiomers is called a Racemic mixture • A racemic mixture is often labeled with a ± in front. Enantiomeric Excess • A sample of an optically active compound that consists of a single enantiomer is said to be enantiomerically pure, or to have an enantiomeric excess(ee) of 100% • %𝑒𝑒 = • %𝑒𝑒 = 𝑚𝑜𝑙𝑠 𝑒𝑛𝑎𝑛 𝐴 −𝑚𝑜𝑙𝑠 𝑒𝑛𝑎𝑛 𝐵 𝑚𝑜𝑙𝑠 𝑒𝑛𝑎𝑛 𝐵+𝑚𝑜𝑙𝑠 𝑒𝑛𝑎𝑛 𝐵 𝑜𝑏𝑠𝑒𝑟𝑣𝑒𝑑 [𝛼] x 100% 𝑝𝑢𝑟𝑒 [𝛼] x 100% Example problem • A sample of 2-butanol showed a [α]=+6.76. What is the actual composition? Synthesis of Chiral Molecules • Reactions using Achiral reactants can often lead to chiral products • In the absence from of any chiral influence from a catalyst, reagent, or solvent, the out come will always be a racemic mixture • Ex. • Reasonings: Stereoselective Reactions • Stereoselective Reactions- are reactions that lead to a preponderance of one stereoisomer over other stereoisomers that could be formed. • If the reaction produces one enantiomer over the other, it is said to be enantioselective. • If the reaction leads to predominately one diastereomer over others that are possible, the reaction is said to be diastereoselective. Stereoselective Reactions • For a reaction to either be enantioselective or diastereoselective, a chiral reagent, catalyst, or sovent must assert an influence on the reaction. • In nature, special proteins called enzymes are used to chirally influence reactions. • Ex. Kinetic Resolution • The previous example of hydrolysis of an ester is an example of Kinetic Resolution • Kinetic Resolution- he rate of a reaction with one enantiomer is different than with the other, leading to a preponderance of one product stereoisomer. Molecules with more than one Stereocenter • Ex. 2,3-dibromopentane • 2n rule- the total number of stereoisomers will not exceed 2n where n= the number of tetrahedral stereogenic centers • Note: the rule states, “will not exceed”; not that there will be that many! Writing the Structures • Begin by writing a 3D formula for one stereoisomer, then draw its mirror image. • Use the eclipsed conformation so that it is easier to identify planes of symmetry • Keep the longest C chain on the vertical axis so the structures are directly comparable • Label the pairs of enantiomers and diastereomers. Diastereomers • Remember, Diastereomers are stereoisomers that are not mirror images. • They also have different physical properties such as MP, BP, solubility, and so on. Back to multiple stereocenters • A structure with 2 stereogenic centers does not always have 4 stereoisomes. • Sometimes there is only 3. • This happens because some molecules are achiral even though they contain stereogenic centers. • Ex 2,3-dibromobutane Meso Compound • Meso Compound- An optically inactive compound whose molecules are achiral even though they contain stereogenic centers. Practice Problems • Practice problems 5.20, 5.21, and 5.22 on pages 220 and 221 are very good practice problems! Nomenclature • For naming, we do the same thing with molecules that have multiple centers as we did with molecules that just had one. • Just take assign a configuration to each center one at a time. Fisher Projections • These are basically a shorthand for what we have been drawing. • Bond orientations are implied so you have to make sure you follow the rules: – The carbon backbone is the vertical line – Vertical lines are going back, while horizontal lines are coming out – Stereogenic Carbons are implied where lines cross – Can NOT flip the projections over, only allowed to rotate them Stereoisomer of Cyclic Compounds • 1,2-dimethylcyclopentane – 2 enantiomers, 1 meso • 1,4-dimethylcyclohexane – No stereogenic centers! – Can exists as cis/trans which are diastereomers, but both are achiral and optically inactive • 1,3-dimethylcyclohexane – 2 enantiomers, 1 meso Stereoisomer of Cyclic Compounds • 1,2-dimethylcyclohexanes – Trans has a pair of enantiomers – Cis is a special case, the enantiomers are actually conformational stereoisomers! Can be interconverted by ring flip Read section 5.15, page 227 • Relating configuration through reactions in which no bonds to the chirality center are broken Resolution • Resolution- the process of separating enantiomers from one another. • We have already seen Kinetic Resolution • Pasteur’s way • Resolution via Diastereomers • Resolution visa interaction with another chiral media.