Chemical Names & Formulas: IUPAC, Ionic Compounds
advertisement

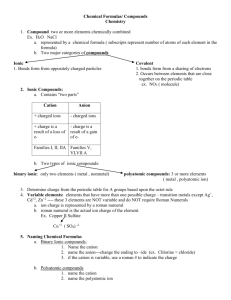
TOPIC 3: CHEMICAL REACTIONS 3.3 IUPAC Chemical Names and Formulas International Union of Pure and Applied Chemistry → standardized naming system (nomenclature) for every known substance Compounds substances composed of two or more different elements held together by chemical bonds. Compounds have no overall charge (neutral charge). Compounds are generally classified as being either ionic or molecular. Terms Binary 2 elements only IONIC COMPOUND compound made of cations (usually metals) and anions Multivalent Metal metal ions that have more than one possible charge Oxidation State actual or apparent charge on an atom in a compound → indicated using Roman numerals when naming Polyatomic “many atoms” → more than 2 elements Acid ionic compound in solution, where cation = H+ MOLECULE compounds made of ONLY non-metal atoms Traditional common, non-systematic (IUPAC), names Organic all carbon containing compounds with a few important Hydrocarbons contain ONLY hydrogen and carbon atoms exceptions Na2O N2O4 sodium oxide dinitrogen tetroxide Cu+ Cu2+ copper (I) ion copper (II) ion Fe2+ Pb4+ iron (II) ion lead (IV) ion NH4Cl CaCO3 ammonium chloride calcium carbonate HCl H2SO4 hydrochloric acid sulfuric acid H2O NH3 water ammonia C6H12O6 CH3CH2OH glucose ethanol CH4 C3H8 methane propane 1.3.1 Write the chemical formula and name for ionic compounds, including transition metals with more than one oxidation state I Ionic Compounds CATION + ANION General Rule for Naming Name of cation + name of anion General Rule for Writing Formula Total Positive Charge = Total Negative Charge Use the lowest whole number ratio of ions that has no net charge Subscripts = number of each ion; do not show “1” Do NOT use prefixes Type 1. Binary ionic compound (without transition metals) CHEMICAL NAME from the Chemical Formula → name cation then anion AlCl3 → Al Cl → CaO → Ca O → aluminum chloride calcium oxide FORMULA from the Chemical Name → balance charges! CHEMICAL NAME CATION + ANION CHEMICAL FORMULA Ions with charges sodium chloride Na+ Cl– magnesium nitride Mg2+ N3– balance charges (Na+) + (Cl–) = 1(+1) + 1(– 1) = 0 (Mg2+) + (N3–) = 3(+2) + 2(–3) chemical formula in lowest form Na1Cl1 = NaCl (Mg2+)3(N3– )2 = Mg3N2 = (+6) + (–6) = 0 Type 2. Multivalent Metals Ag and Zn A few transition metals have only one possible charge (oxidation) → Do NOT use Roman numerals o silver ions are ALWAYS +1 Ag+ AgCl = silver chloride o zinc ions are ALWAYS +2 ZnCl2 = zinc chloride Fe, Cu, Sn, Pb Zn2+ → Multivalent Metals that have more than one possible oxidation state → NEED Roman numerals to show their CHARGE CHEMICAL NAME Determine its CHARGE from the formula Write the CHARGE in parentheses using Roman numerals o 1+ → (I) 2+ → (II) 3+ → (III) 4+ = (IV) CHEMICAL FORMULA Balance charges (add to 0) Cation CHEMICAL NAME CATION + ANION FeO (Fex)1 (O2–)1 1(x) + 1(2–) = 0 →x = 2+ Fe2+ iron (II) oxide Fe2O3 (Fex)2 (O2–)3 2(x) + 3(2–) = 0 2x – 6 = 0 → x = 3+ Fe3+ iron (III) oxide FORMULA from the Chemical Name → same as regular binary ionic compounds balance charges! CHEMICAL NAME CATION + ANION lead (II) nitride tin (IV) sulfide CHEMICAL FORMULA Ions with charges Pb2+ Sn4+ N3– S2– balance charges (Pb2+) + (N3–) = 3(+2) + 2(–3) = (+6) + (–6) = 0 (Sn4+) + (S2–) = 1(+4) + 2(–2) = (+4) + (–4) = 0 chemical formula in lowest form (Pb2+)3(N3– )2 = Pb3N2 Sn1S2 = SnS2 Type 3. Polyatomic Ions Polyatomic ions are two or more atoms that act as a group and share a total charge. KEEP THE POLYATOMIC GROUPS TOGETHER – DO NOT CHANGE SUBSCRIPTS use parentheses when showing more than one ion present in formula Your data sheets have the formulas and charges of the common polyatomic ions You are expected to be able to name and use these ions. Polyatomic Cation there is only 1 NH4+ ammonium ion Polyatomic Anions – most are combinations of oxygen with another element HYDROXIDE OH– -ATES CO23 carbonate calcium carbonate = CaCO3 NO-3 nitrate aluminum nitrate = Al(NO3)3 € PO3-4 phosphate sodium phosphate = Na3PO4 € SO2-4 sulfate copper (II) sulfate = CuSO4 € € CO23 nitrate ion SiO2-4 PO3-4 silicate ion CHEMICAL NAME magnesium hydroxide = Mg(OH)2 NO-3 € carbonate ion € SO2-4 phosphate ion sulfate ion ClO-3 chlorate ion Hint: Circle any polyatomic anions in the formula € Change –IDE (I Don’t have € Oxygen) to –ATE (except for € OH = hydroxide) € sodium hydroxide = NaOH FORMULA from the Chemical Name → use (parentheses around groups) when balancing charges! CHEMICAL NAME CATION + ANION iron (III) nitrate potassium sulfate ammonium sulfide CHEMICAL FORMULA Ions with charges Fe3+ K+ NH4+ NO3– SO42– S2– balance charges (Fe3+) + (NO3–) = 1(+3) + 3(–1) = (+3) + (–3) = 0 (K+) + (SO42–) = 2(+1) + 1(–2) = (+2) + (–2) = 0 (NH4+) + (S2–) = 2(+1) + 1(–2) = (+2) + (–2) = 0 chemical formula in lowest form (Fe3+)1(NO3– )3 = Fe(NO3)3 (K+)2(SO42–)1 = K2SO4 (NH4+)2(S2–)1 = (NH4)2S