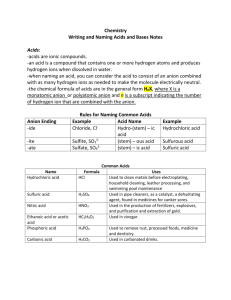
Chapter 19 Acids and Bases GRQ Sulfuric acid is used in the
advertisement

Chapter 19 Acids and Bases GRQ 1. Sulfuric acid is used in the manufacture of products such as _plastics__, detergents___, _batteries__, and _metals___. 2. Acidic solutions taste sour. 3. Give 3 examples of acids and what they are responsible for. 1. Carbonic and phosphoric acids give many carbonated beverages their sharp taste. 2. Citric and ascorbic acids give lemons and grapefruit their mout-puckering tartness. 3. Acetic acid makes vinegar taste sour. 4. 5. 6. 7. Basic solutions taste bitter and feel slippery. Litmus is a dye commonly used to distinguish solutions of acids and bases. Are neutral solutions acidic or basic? neither What determines whether an aqueous solution is acidic, basic, or neutral? The relative amounts of H+ and OH- ions 8. Does a neutral solution contain more hydroxide or hydrogen ions? Neither, contains equal amts. 9. What is self-ionization? Two water molecules react to form a hydronium ion (H30+) and a hydroxide (OH-) ion 10. What is a hydronium ion? A hydrated hydrogen ion (water molecule attached to a hydrogen bond by a covalent bond 11. Define an acid using the Arrhenius model. A substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous soln 12. Define a base using the Arrhenius model. A substance that contains a hydroxide group and dissociates to produce a hydroxide ion in aqueous soln 13. How does the Bronsted-Lowry model define acids and bases? Acid: hydrogen-ion donor base: hydrogen-ion acceptor 14. Give a reaction and label the acid, conjugate acid, base, and conjugate base. NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) Base Acid Conj acid Conj base 15. Define amphoteric. Substance that are able to act as either an acid or a base 16. Give an example of a monoprotic acid. An acid that can donate only one hydrogen ion a polyprotic acid acids containing more than one ionizable hydrogen atom 17. What is a strong acid? Acids that ionize completely 18. Name 6 strong acids and give their chemical formulas. a. hydrochloric (HCl) d. perchloric (HClO4) b. hydrobromic (HBr) e. nitric (HNO3) c. hydroiodic (HI) f. sulfuric (H2SO4) 19. What is a weak acid? Acid that ionizes only partially inn dilute aqueous soln 20. Name 6 weak acids and give their chemical formulas. a. hydrofluoric (HF) d. hydrosulfuric (H2S) b. hydrocyanic (HCN) e. carbonic (H2CO3) c. acetic (HC2H3O2) f. hypochlorous (HClO) 21. Strong acids produce weak conjugate bases and weak acids produce strong conjugate bases. 22. Strong bases produce weak conjugate acids and weak bases produce strong conjugate acids. 23. What is a strong base? Bases that dissociate entirely into metal ions and hydroxide ions 24. Name 6 strong bases and give their chemical formulas. a. sodium hydroxide (NaOH) d. cesium hydroxide (CsOH) b. potassium hydroxide (KOH) e. calcium hydroxide (Ca(OH)2) c. rubidium hydroxide (RbOH) f. barium hydroxide (Ba(OH)2) 25. What is a weak base? Ionizes only partially in dilute aqueous soln to form conj acids of the base and hydroxide ion 26. Name 5 weak bases and give their chemical formulas. a. methylamine (CH3NH2) d. aniline (C6H5NH2) b. ethylamine (C2H5NH2) e. c. ammonia (NH3)