Solutions-Acids-Bases Study Guide - Chemistry
advertisement

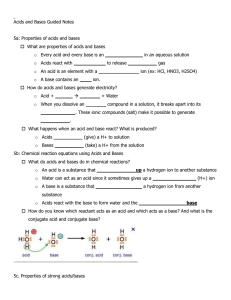
Solutions – Acids and Bases Study Guide Solutions: You should know- Solute Solvent Solution Supersaturated Unsaturated Saturated Miscible Immiscible Solubility How to read a solubility graph Formula for concentration and how to solve concentration problems Heating and cooling curves Phase change diagrams Acids and Bases - Arrhenius Theory Bronsted- Lowry Theory Acids produce H+ ions o Know characteristics of acids - Bases produce OH- ions - - - o Know characteristics of bases How to determine conjugate acids and conjugate bases How to write neutralization equations o What do they ALWAYS produce? o What is a salt? pH, pOH, hydrogen ion concentration, hydroxide ion concentration o how are theses related o what is the pH scale o if you have one value, how can you solve for the others? Titration problems o Solve for unknown concentration of acid or base Which substance is least soluble at 50oC? What is the solubility of NH4Cl at 70oC? Which substance does heat have the greatest effect on? Name some strong acids. Name some strong bases. Neutralization reactions always produce what 2 substances? Acids are associated with ____________________ ions. Bases with _______________________ ions. List the pH of the following substances: 0.001M HBr 0.0001M KOH 1 x 10-11M H2SO4 1 x 10-4 M NaOH 1.00 HNO3 hydroxide ion concentration of 1 x 10-13