Acids and Bases 2
advertisement

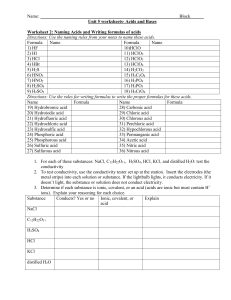
Acids and Bases 2 Chemical Formula: Acids When certain chemicals are mixed with water they behave like acids: eg. HCl We need to show that these chemicals are mixed with water when we are using them so we write it as HCl(aq) (aq) aqueous = dissolved in water to make a solution Writing chemical formulas for acids Formulas for Acids: – Usually have the Hydrogen first (HCl(aq) , HNO3(aq)) – When carbon is also in the formula then the H is written on the end (CH3COOH(aq) ) Writing Chemical Formulas/Names Formulas for Acids: – Usually have the Hydrogen first (HCl(aq) , HNO3(aq)) – When carbon is also in the formula then the H is written on the end (CH3COOH(aq) ) Names for Acids: • NO (AQ) HCl – Usually the hydrogen goes first (Hydrogen chloride) • (AQ) HCl(aq) – May have a different name that ends in “-ic acid” Common acids FORMULA HCl(aq) H2SO4(aq) CHEMICAL NAME COMMON NAME EXAMPLE OF USES Hydrochloric Muriatic Stomach acid, breaks down acid acid food Sulfuric acid Battery acid Metal cleaner, battery acid HNO3(aq) Nitric acid Nitric acid CH3COOH(aq) Ethanoic acid Acetic acid Makes fertilizer Found in vinegar Writing chemical formulas for Bases Formulas for Bases: – Usually has a hydroxide (OH) eg. NaOH – Same as any ionic compound (Sodium hydroxide) Common bases FORMULA CHEMICAL NAME COMMON NAME EXAMPLE OF USES NaOH Sodium hydroxide Magnesium hydroxide Caustic soda Milk of magnesia cleaner Calcium hydroxide Ammonium hydroxide Hydrated lime Ammonia Mg(OH)2 Ca(OH)2 NH4O H antacids Soil and water treatment Kitchen cleaner ions • Acids = produce ions (H+) when dissolved in water A high concentration of H+ = low pH • Bases = produce ions (OH-) when dissolved in water A high concentration of OH- = high pH Why is water neutral? H+ + OH- H2O (H-OH) A water molecule is made of one H (acid ion) and one OH (base ion) = acids and bases are balanced