Acid Base and Gas Forming Reaction Practice
advertisement

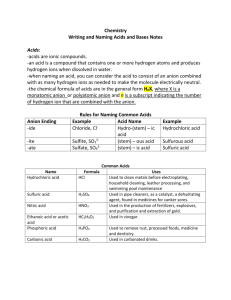
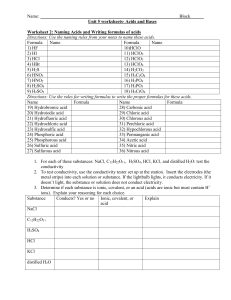
AP Chemistry 2015-2016 Acid-Base & Gas-Evolution Reactions Name: Date: Per: Directions: Write the balanced molecular equation, full ionic equation, and net ionic equation. Solutions of Strong Acids & Strong Bases 1. Solutions of hydrochloric acid and barium hydroxide are mixed. 2. Solutions of sulfuric acid and potassium hydroxide are mixed. Solutions containing Weak Acids and/or Weak Bases Remember that weak acids and weak bases are weak electrolytes, meaning they do not fully ionize in water. So when you write your full ionic equation, you cannot pull them completely apart. Example: acetic acid reacts with sodium hydroxide Molecular: HCH3COO (aq) + NaOH -> NaCH3COO (aq) + H2O (l) Full ionic: HCH3COO (aq) + Na+ (aq) + OH- (aq) -> Na+ (aq) + CH3COO- (aq) + H2O (l) Net ionic: HCH3COO (aq) + OH- (aq) -> CH3COO- (aq) + H2O (l) 3. Solutions of calcium hydroxide and carbonic acid are mixed. 4. Solutions of hydrobromic acids and ammonium hydroxide are mixed. Solutions reacting with Solids 5. Solid potassium hydroxide is added to a solution of sulfuric acid. 6. A solution of hydrochloric acid is added to solid copper(II) hydroxide. Gas Forming Reactions 7. A solution of ammonium sulfate is added to solid lead hydroxide. 8. Solid iron(III) sulfide is added to a dilute solution of sulfuric acid.