Cnidarians and sponges cont.
advertisement

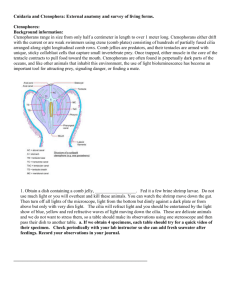
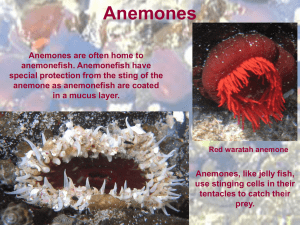
Today you will continue your observations of Porifera and Cnidaria and view specimens of Ctenophora: 1. Porifera: Before tackling new material, examine your sponge cultures. Take another photograph at low and high power. Compare your photographs in your journal from those taken of the new cultures last week. If your culture failed and some do despite all precaution, please use one of your classmates photographs that was more successful. We are receiving some material, the day of the lab. If it does not arrive in time for the morning lab, we need to adjust schedule so look at the table below and also listen carefully for your laboratory instructions. Scenario one: All specimens expected arrive. One pair at table, do ctenophores, a One pair at table do mapping of Hydractinia echinata colonies on hermit crabs. b Pairs switch Every one does classroom exercise on anemones and nematocysts. c One pair compares octocorals to anemones., One pair review pennaria from last week and d compare new hydroid to it. e Pairs switch Scenario two: If Hydractinia echinata does not come in. . One pair at table, do ctenophores One pair compares octocorals to anemones. One pair review Pennaria from last week and compare new hydroid to it Pairs switch C. Every one does classroom exercise on anemones and nematocysts a. Ctenophores Background information: Ctenophorans range in size from only half a centimeter in length to over 1 meter long. Ctenophorans either drift with the current or are weak swimmers using ctene (comb plates) consisting of hundreds of partially fused cilia arranged along eight longitudinal comb rows. Comb jellies are predators, and their tentacles are armed with unique, sticky colloblast cells that capture small invertebrate prey. Once trapped, either muscle in the core of the tentacle contracts to pull food toward the mouth. Ctenophorans are often found in perpetually dark parts of the oceans, and like other animals that inhabit this environment, the use of light bioluminescence has become an important tool for attracting prey, signaling danger, or finding a mate. The light observed in this species is actually from the movement of the ctenes which refracts light as the animal moves. Obtain a dish containing a comb jelly . Fed it a few copepods or other food available. They may not feed. Do not use much light or you will overheat and kill these animals. You can watch the shrimp move down the gut. Then turn off all lights of the microscope, light from the bottom but dimly against a dark plate, or from above but only with very dim light. The cilia will refract light and you should be entertained by the light show of blue, yellow and red refractive waves of light moving down the cilia. These are delicate animals and we do not want to stress them, so keep the lights low unless filming and try not to overfeed. Each pair should try for a quick movie of their specimen either feeding or just record the light show caused by their movement. Check periodically with your lab instructor so she can add fresh seawater after feedings. Record your observations in your journal. ___________________________________________________________ b. Obtain a hermit crab or at least a shell containing a colony of Hydractinia echinata. Identify if you can at least four types of individuals. Draw the different individuals you see. Graph the colony indicating the position of the various individuals to each other. Do not attempt in this drawing to show each individual but do indicate relative numbers and overall pattern. Watch carefully for epifauna. Various tubed annelids often live among the hydroids. Although considered a plus for the annelids that are protected, this relationship may be a minus for the hydroid colony as I have seen the worms steal food from the hydroids. Also look for other hydroids that may be competing with Hydractinia echina for space. Colonies that have to share their shell with these competitors may have different numbers of different individual or place their individuals in different positions relative to the intruding species. If you had to design a colony where would you place reproductives relative to defense individuals? Would the placements vary dependent on whether competitors were present or absent? Colonies are often male or female (or more strictly speaking have only male or female gonozoids). Can you tell whether the colony on your shell is a male or female colony? Construct hypotheses regarding different conditions and then see if the distribution supports your hypotheses. Each pair should collect data on one shell. Another diagram that divides the colony into non reproductive zooids and female and male reproductive zooids. Shells for your to use to plot distribution of different zooids. Choose the shell shapes that best fit the shell of your crab. Shape one: Most common shape Are there any polyps on the underside of the shell? Use the diagram below. Shape two, front, side views and ventral (bottom) of shell Shape three: Exercise four Hydractinia echinata _________________________________________________________________________ Exercise 5-6: We do have several species of Anthozoa: While many members of the phylum Cnidaria have a life cycle that includes both medusoid and polyp stages, sea anemones are exclusively polyp. You will work closely with a sea anemone and then examine two species of octocorals. Exercise c: Sea Anemones: The external morphology of anemones is limited to a column; an oral disk, in the center of which the mouth is located, and on which the tentacles are located; and either a pedal disk, that affixes the anemone to the substrate, or a bulb-like physa, used by burrowing anemones to anchor in soft substrate. . Aiptasia pallida, or related species, has long tentacles and demonstrates partial retraction. Because they reproduce by budding, a few well-fed individuals will soon multiply to hundreds and cover the rocks like soft brown fuzz. Obtain and examine a specimen of Aiptasia pallida. This anemone, contains symbiotic dinoflagellates or unicellular algae. Apitasia is fairly transparent and so you should be able to see the food moving down into the gut. It feeds on most motile organisms. It will often take animals twice or three times its size. Feed your specimen a small piece of fish food. How close is the prey before the anemone response to it. Do the tentacles move toward the prey or does the prey have to contact the tentacles before the anemone responds. How does the sea anemone move the prey animal into the digestive cavity? Can you see any other details of internal anatomy in your specimen? Record your observations in your journal. Pairs should exchange information and then all should be able to look at cnidoblast cells at the same time. Your teaching assistant will choose a specimen to use to examine the stinging cells or cnidoblasts. Obtain a tentacle or two and place under 100 or 200 x. Look closely at the surface to see the conspicuous spherical nematocysts. These are the explosive capsules of cnidocytes. The cnidocytes themselves will probably not be discernable but their capsules are abundant and conspicuous. You may also see some yellow to yellow orange circular cells that are spilling out of the tentacles. These are symbionts, or dinoflagellates found in this species. Some pairs may want to place the acontia or white protective threads that appear when the anemone feels threatened, (as when its tentacles are cut off). Anemones can have cnidocytes lining the inside of the gastrovascular cavity and their acontia. Look at one of the tentacles with 400X. Note the different sizes of nematocysts. Most of the nematocysts will be intact and unexploded but some will have discharged. Find some of the large ones, focus carefully, and look for a coiled thread inside the capsule. If the thread is present, the nematocyst is undischarged. Look around for some discharged nematocysts. These will look quite different. They are obviously empty, having everted their thread, which can be clearly seen extending away from one end of the empty capsule. With careful focusing and light adjustment you can also see the formidable barbs at the base of the thread adjacent to the empty capsule. Place a drop of 1% acetic acid beside the coverslip and draw it under while watching through the microscope. The acid may stimulate the discharge of many of the nematocysts and, if you are fortunate, you may actually see one of them discharge as you watch. A drop of toluene blue applied the same way will stain the nematocysts, making them easier to see. Obtain (a.) a photograph of a tentacle, discharged nematocysts and symbionts. Identify symbiotic algae and nematocysts on the photograph. Exercise d Included in the Anthozoa are the true corals. We usually do not get representatives of the true corals but closely related to them are the gorgonians. Gorgonians, Gorgonians and sea pansies are also known as octocorals, so named because the individual polyps have eight tentacles that they use to feed. If you look closely at the branches of a soft coral (gorgonians are probably the most obvious), you may see the tiny tentacles of the individual polyps. Each of these tentacles may looks like it is feathered because it can bears numerous outfoldings. The main purpose of this exercise is for you to observe these animals and be able to compare them to the hydroid colonies you observed previously (Hydractinia and any hydroids available today), Many gorgonians, sea pansies or pens look superficially like hydroid colonies, but on close examination, you can see their more “sea anemone” or anthozoan morphology. One of the lab questions on the next exam will focus on distinguishing between the two. -----------------------------------------------------------------------------------------Available Octocorals Please examine two specimens. Leptogorgia virgulata This is also a tree-shaped gorgonian with colorful ( colors can range from tan to purple) branches rising in all directions from a short, main trunk Parazoanthus sp. these are large sponge zooanthids that grow symbiotically with sponge species. Renilla mulleri, a sea pansy, This colony looks like a bit of a sponge with fuzz on it. Polyps are vividly luminescent when handled in the dark. It as the sea pen has a peduncle or base that anchors it to the substrate. --------------------------------------------------------------------------------------------------------------------Look at a portion of the available Gorgonian colony and a Sea pansy or fan under the dissecting scope. Be patient as it may take a while for the individuals to relax and expand. Focus on the tentacles and see if you can observe their “pinnate” nature. Count number of tentacle, etc. a. Obtain a photograph and indicate “octocoral” features, b. Compare the structure of these colonies to that of an hydrozoan colony and Aiptasia in your journal. ___________________________________________________________________ e. Cnidaria: Colonies of various hydrozoans. 1. You will continue to look at variation among the class Hydrozoa. In all specimens examined, you should determine (if only from the web) what life stages to expect and whether the colonies exhibit polymorphisms. Each table should compare 2 hydroids Use Pennaria as your first specimens. Then compare overall growth form to either Campanullaria or Sertularia. Pennaria is the hydroid you looked at last week, so if those photographs are good use them. a. Take photographs of the different types of polyps present. In your journal compare and contrast the different types of polyps found. It looks like in Tubularia, feeding polyps prevail and in Pennaria, reproductive individuals prevail. It looks like some of the reproductive polyps are developing medusa, although medusa are far from being released. If medusa are present be careful, use gloves, as all medusae sting, although most of the available species, produce medusa so small, you probably will not be able to feel their sting. b. Film feeding in Pennaria c . Characterize the shape of the colony and spacing of feeding versus other types of zooids. Are all the gonozoids at the colony edges or in the middle of the colony? Are zooids only found at the terminal tips of hydroids or are they spaced regularly on the branching stolons. Typical life stages of hydroids Specimens Pennaria spp In Pennaria tiarella, medusa buds develop and are retained along sides of hydranths.. Generally colonies are male or female, although the medusae of both sexes look alike and are difficult to distinguish. In other species, medusa may be released and develop from gonophores produced at the edges of branches. Sertularia This species is often limited in nature to area of strong flow. he main stem gives rise to side branches of determinate length which branch dichotomously. They bear hydrothecae which are arranged subalternately along opposite sides of the branches, turned slightly towards the distal end of the colony. The outer part of each hydrotheca is turned slightly away from the branch. Gonothecae are borne abundantly on these side branches Obelia or Campanullaria. Obelia forms a long white branching colony more than ten centimeters long. The polyps of Obelia are housed in transparent cups for protection. When touched or exposed to the microscope's light the tentacles will withdraw into the cups. This species is the only one in the laboratory that will produce obvious medusae, which are released from the gonozooids, producing free-swimming male and female medusae velum with gonads, a mouth, and tentacles. The physical appearance of the male and female medusae velum, including their gonads, are indistinguishable, and the sex can only be determined by observing the inside of the gonads, which will either contain sperm or eggs. The medusae reproduce sexually, releasing sperm and eggs that fertilize to form a zygote, which later morphs into a blastula, then a ciliated swimming larva called a planula. Campanularia sp. May be substituted for Obelia. Polys look that same. They are in the same family, but medusa are different, Campanularia being more boxlike.