Cnidaria
advertisement

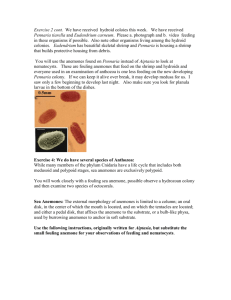
Cnidaria We have obtained healthy but small specimens of some species this year. We should have enough specimens for one pair at a table to engage in activity one and the other in activity two. Once done, switch specimens with the other pair of students. That way. no one should have to wait for specimens. Activity 1. The species that was to be our model Pennaria tiarella is unfortunately just getting started. You will be able to use it to examine the structure of a simple hydroid colony and more importantly the other animals a colony of this sort attracts. One of the attractions in this colony is skeleton shrimp. Everyone pair should obtain a movie of skeleton shrimp in motion and compare their locomotion to other crustaceans in your journal. Again you will be adding observations on different species to your journal entries on arthropod behavior throughout the term. Remember arthropods are everywhere. What other species can you find within these colonies? Species belonging to the class Hydrozoa contain cnidarians with some of the simplest structure found in the clade, Cnidaria. At the least. colonies consist of feeding polyps connected to each other by stolons. Your colony is just coming out of dormancy. The orange structures within zooids are developing embryos. They will hatch out as planula larvae and form new colonies. _______________________________________________________ I isolated some planula larvae from the dying hydroid colony (unknown species) that was attached to the Barnacles two weeks ago. A few of them have initiated colonies on one of our large dishes. The individuals who use this dish to view feeding polyps need to share their observations with the class. Obtain photographs of your colony and compare it to the diagram below of a colony with well developed feeding polys (If we are lucky and can isolate planula from this colony, hopefully what our new colonies will look like in a few weeks). In Pennaria tiarella, medusa buds develop along sides of hydranths. Be careful, use gloves, Pennaria medusae as all medusae sting. Generally colonies are male or female, although the medusae of both sexes look alike Activity two. Overall structure of a feeding polyps. Most hydrozoan colonies consists of feeding individuals connected to each other by a stolon. Most colonies at certain times of the year will produce reproductive polyps. These may contain both or more likely either sperm producing or egg producing structures. There are four specimens available. 1-3 There are 3 colonies of Hydractinia that contain feeding polyps (this is the species on the hermit crab shells). 4. Specimens of an unknown colony that came in with the barnacles is also available. These feeding polyps are the first to develop from planula larvae that were shed by the colony. These specimens will allow four tables to observe feeding individuals at one time. Make observations on the overall structure of your specimens. Focus on a feeding polyp and notice its tentacles. Try to feed your polyps. Put a small sample of brine shrimp, copepods or rotifers in the plate. If the colony does not seem interested, try some refrigerated food, phytoplankton or micro-zoo plankton. You should at lest be able to record the response of feeding polyps which should extend their body and lengthen their tentacles in an effort to locate the food source. Hydractinia If time permits, for your Hydractinia colony Identify if you can at least four types of individuals. Hydractinia has defensive stinging and structural (spike like to discourage settling of other species) polyps as well as reproductive and feeding polyps. Next week you will attempt to graph their position on the shells. _______________________________________________ Tables can divide themselves again into pairs with one pair initially tackling activity three and the other activity four. Activity three: Obtain a transparent specimen of Aiptasia pallida. Aiptasia is a anemone that aquarium keepers dread. It quickly takes over the tank, killing all other sessile organisms and feeding on most motile organisams. It will often take animals twice or three times its size. Try feeding fish food, or brine shrimp to specimens.. Lighter or white specimens are almost transparent and you can observe the food move through the gastroventricular cavity or coelenteron, as it is know in this group. How close is the prey before the anemone response to it? Do the tentacles move toward the prey or does the prey have to contact the tentacles before the anemone responds. How does the sea anemone move the prey animal into the digestive cavity? Can you see any other details of internal anatomy in your specimen? Anemones not only have cnidocytes on their tentacles, but internally lining the gut and on acontia that are released when these animals fight over space or are threatened by a predator. Need we re-state, anemones are awesome predators. Can you can see the shrimp or fish food traveling down the gastrovascular cavity? Next week, you will be able to examine some slides that will illustrate that these species rank among the most complex structurally of the Cnidarians. Activity four. Gorgonians, Leptogorgia virgulata and Sea pansies, Renilla mulleri. Gorgonians and sea pansies are also known as octocorals, so named because the individual polyps have eight tentacles that they use to feed. If you look closely at what appears to be “holes” on the surface of one of these, you may see the tiny tentacles of the individual polyps. Each of these tentacles may looks like it is feathered because it can has numerous outfoldings. Look at a portion of the available Gorgonian colony or a Sea pansy under the dissecting scope. Be patient as it may take a while for the individuals to relax and expand. Focus on the tentacles and see if you can observe their “pinnate” nature. Each group should choose to film the gorgonian or one of the sea pansies available. Compare the structure of their polyps with those in a hydroid colony. These species are more complex and in the same group as true corals and sea anemones, the Anthozoa. _________________________________________________ Pairs should exchange information with regard to activity 3 and 4 and then the class as a whole should be able to look at cnidoblast cells at the same time. Obtain a light microscope for each table. Make sure your scope has a camera attached. Each pair should make a slide and then share their observations and photos with the other pair of students at a table. You will have to switch the adapter from your stereo scope to your light microscope . Your instructor will show you how. Make sure your adapter goes back on the stereoscope cable once you are done with activity five. Activity five Your teaching assistant will choose a specimen to use to examine the stinging cells or cnidoblasts. Obtain a tentacle or two and place under 100 or 200 x. Look closely at the surface to see the conspicuous spherical nematocysts. These are the explosive capsules of cnidocytes. The cnidocytes themselves will probably not be discernable but their capsules are abundant and conspicuous. You may also see some yellow to yellow orange circular cells that are spilling out of the tentacles. These are symbionts, or dinoflagellates found in this species. Some pairs may want to place the acontia or white protective threads that appear when the anemone feels threatened, (as when its tentacles are cut off). Anemones can have cnidocytes lining the inside of the gastrovascular cavity and their acontia. Look at one of the tentacles with 400X. Note the different sizes of nematocysts. Most of the nematocysts will be intact and unexploded but some will have discharged. Find some of the large ones, focus carefully, and look for a coiled thread inside the capsule. If the thread is present, the nematocyst is undischarged. Look around for some discharged nematocysts. These will look quite different. They are obviously empty, having everted their thread, which can be clearly seen extending away from one end of the empty capsule. With careful focusing and light adjustment you can also see the formidable barbs at the base of the thread adjacent to the empty capsule. Place a drop of 1% acetic acid or some brine shrimp juice (crush some brine shrimp on a separate slide, use the fluid from that slide) or shrimp juice beside the coverslip and draw it under while watching through the microscope. The acid may stimulate the discharge of many of the nematocysts and, if you are fortunate, you may actually see one of them discharge as you watch. A drop of toluene or methyl blue applied the same way will stain the nematocysts, making them easier to see. Take a photo of your prep, labeling cnidocytes and discharged nematocysts. Make sure you use your thumb drive to transfer the photos to the computer containing your films, etc. if the microscope happens to be connected to another computer. Please leave your slides for me to photograph at night. I can find some oil and so obtain some very high resolution shots to share with the class.