ADE-13 Hypoglycemia in Patients on Insulin - K-HEN
advertisement

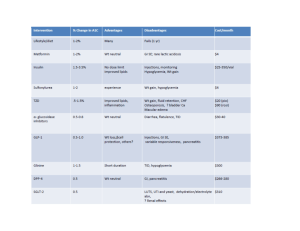
Measure Information Measure Topic: Adverse Drug Event (ADE) Measure ID: EOM-ADE-13 Measure Name: Hypoglycemia in inpatients receiving insulin Description: Hypoglycemia in inpatients receiving insulin or other hypoglycemic agents Rationale: Medications are the most common intervention in healthcare but are also most commonly associated with adverse events in hospitalized patients. At least 20% of all harm is associated with medication errors. High-alert medications are more likely to be associated with harm than other medications; they cause harm more commonly, the harm they produce is likely to be more serious, and they “have the highest risk of causing injury even when used correctly.” Insulin, anticoagulants, narcotics and sedatives are the medications responsible for the majority of harm due to high-alert medications. Type of Measure: Outcome Improvement Noted As: A decrease in the rate Numerator Statement: Hypoglycemia occurrences in inpatients receiving insulin. Hypoglycemia is defined as a plasma glucose concentration of 50 mg per dl of less. Included populations: All inpatients with a plasma glucose concentration of 50 mg/dl Excluded populations: Blood glucose drawn prior to admission (e.g. in the Emergency Department prior to admission) Denominator Statement: Inpatients receiving insulin therapy Included populations: Inpatients admitted to the hospital Excluded populations: Outpatients Risk Adjustment: No Data Collection Approach: Concurrent review of all inpatients with a blood glucose level less than or equal to 50 mg/dl is ideal. When not feasible to perform concurrent review, retrospective chart review may be substituted. Data Accuracy: Not applicable Measure Analysis Suggestions: The organization should have an active ongoing process for detecting, reporting and assessing adverse patient events related to insulin use. Organizations should monitor the use of 50% dextrose and glucagon as triggers for case review to determine if that use was associated with insulin therapy. Review numerator cases for symptoms such as lethargy and shakiness documented in nursing notes, and the administration of glucose, orange juice, or other intervention. If symptoms are present, look for associated use of insulin or oral hypoglycemics. Evaluate timing of medication administration in conjunction with meal times. Strategize improvements to minimize the occurrence of hypoglycemia. Sampling: Yes, if number of patients is greater than 20 per month Sampling Strategy: Review a minimum of 20 records per month from the population of patients receiving insulin. You may conduct reviews over multiple sessions (for example, 10 records every 2 weeks) to spread out the need for resources. If you are beginning this intervention within a pilot unit or units, limit your initial measurement to only those units. As you spread the implementation, expand your measurement accordingly. Select patient records randomly, or in a way that avoids a selection bias. While not completely random, one easy method of chart selection is to print out all admissions in which the patient received at least one dose of insulin and select every 10th chart for review. Data Reported as: Rate (Numerator/Denominator * 100) Selected References: American Society of Health-System Pharmacists; http://www.ashp.org/s_ashp/docs/files/Safe_Use_of_Insulin.pdf Institute for Healthcare Improvement; IHI Global Trigger Tool White Paper2009 http://www.ihi.org/knowledge/Pages/Tools/IHIGlobalTriggerToolforMeasuringAEs.aspx