jcb25075-sup-0001-SuppData_S1
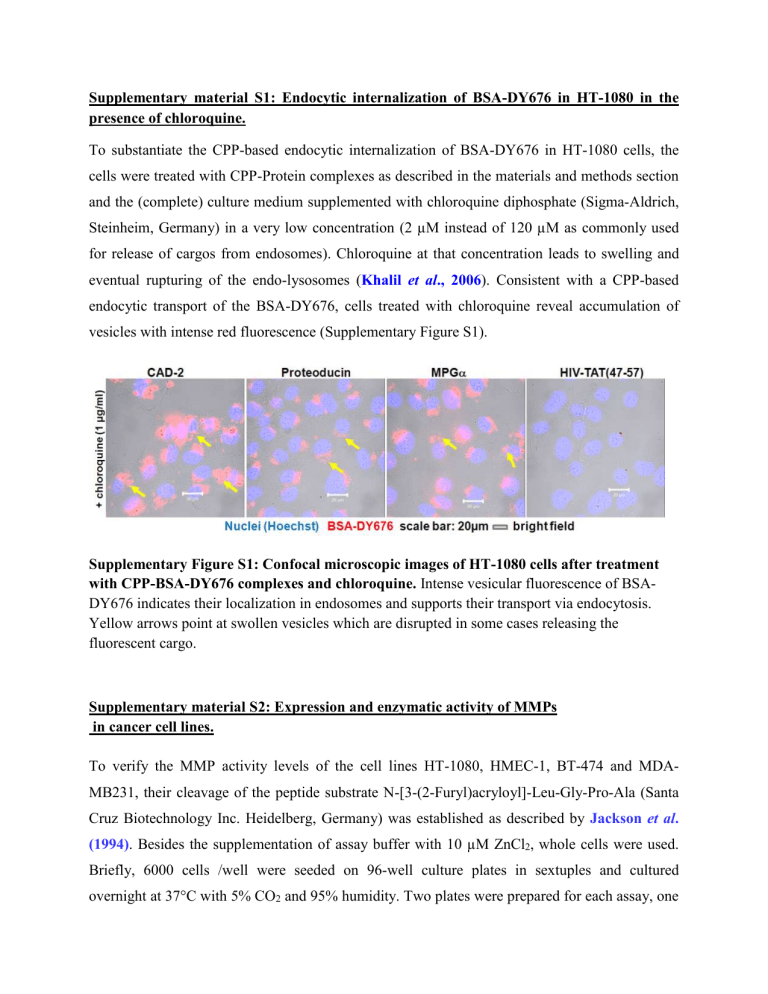
Supplementary material S1: Endocytic internalization of BSA-DY676 in HT-1080 in the presence of chloroquine.
To substantiate the CPP-based endocytic internalization of BSA-DY676 in HT-1080 cells, the cells were treated with CPP-Protein complexes as described in the materials and methods section and the (complete) culture medium supplemented with chloroquine diphosphate (Sigma-Aldrich,
Steinheim, Germany) in a very low concentration (2 µM instead of 120 µM as commonly used for release of cargos from endosomes). Chloroquine at that concentration leads to swelling and eventual rupturing of the endo-lysosomes ( Khalil et al., 2006 ). Consistent with a CPP-based endocytic transport of the BSA-DY676, cells treated with chloroquine reveal accumulation of vesicles with intense red fluorescence (Supplementary Figure S1).
Supplementary Figure S1: Confocal microscopic images of HT-1080 cells after treatment with CPP-BSA-DY676 complexes and chloroquine. Intense vesicular fluorescence of BSA-
DY676 indicates their localization in endosomes and supports their transport via endocytosis.
Yellow arrows point at swollen vesicles which are disrupted in some cases releasing the fluorescent cargo.
Supplementary material S2: Expression and enzymatic activity of MMPs
in cancer cell lines.
To verify the MMP activity levels of the cell lines HT-1080, HMEC-1, BT-474 and MDA-
MB231, their cleavage of the peptide substrate N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala (Santa
Cruz Biotechnology Inc. Heidelberg, Germany) was established as described by Jackson et al.
(1994) .
Besides the supplementation of assay buffer with 10 µM ZnCl
2
, whole cells were used.
Briefly, 6000 cells /well were seeded on 96-well culture plates in sextuples and cultured overnight at 37°C with 5% CO
2
and 95% humidity. Two plates were prepared for each assay, one
being a buffer control where no substrate was added. After culturing overnight, the cells were washed 2 times with MMP assay buffer (50 mM Tricine, 400 mM NaCl, 10 mM CaCl
2
, 10 µM
ZnCl
2
, Sigma-Aldrich, Steinheim, Germany) pH 7.5. The absorbance of the cells were monitored at 305 nm (wavelength for substrate concentration control) and at 345 nm (wavelength at which proteolytically cleaved substrate is monitored) on a Tecan Infinite M1000 plate reader (TECAN,
Crailsheim, Germany). Thereafter, 200 µl of a 300 µM substrate solution in assay buffer was added to the cells and the absorbance was measured immediately at 345nm (t=0) and then every minute for the initial 5 min then again after 23 h. The absorbance of cells in the absence of the substrate was subtracted from the respective samples. Furthermore, the difference in absorbance between time t=0 and t=23 h which correlates with the relative activity of MMPs on the cell surface was deduced and presented in a bar diagram (Figure S2A). All the cell lines revealed only very low levels of the substrate cleavage. However, the HT-1080 cell line revealed a significantly higher cleavage of the substrate ( P = 0.0012) than the other cell lines. This cleavage was comparable to that of 0.125 µg purified MMP-9 protein (R&D systems, Abingdon, United
Kingdom). There was no detectable cleavage of the substrate by whole cells within the initial 5 minutes. Interestingly, cell culture supernatant diluted at 1:4 with assay buffer revealed detectable levels of substrate cleavage within 5 minutes, with HT-1080-derived culture supernatant showing the highest cleavage rate (Figure S2-B). This suggests that the cell lines all express and secrete active MMPs into the culture supernatant. However, it is difficult to say exactly which MMPs are responsible for the cleavage.
Supplementary Figure S2: MMP activity on cell surface and in cell culture supernatant.
(A)
Each bar represents the difference in absorbance (345nm) of intact (t=0) and proteolytically cleaved FALGPA (t = 5 min for culture supernatant / t = 23 h) after subtracting the overall absorbance of the cells in the absence of substrate. MMP9*: Purified MMP9 protein (0.125µg
/assay sample) was used as control. (B) Each bar represents the difference in absorbance (345nm) of intact (t=0) and proteolytically cleaved FALGPA (t = 5 min) after subtracting the overall difference in absorbance caused by the respective culture media in the presence of substrate. Data represent the mean of 2 independent experiments + standard deviation.
References
Khalil IA, Kogure K, Akita H, Harashima H. 2006. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev 58 : 32-45.
Jackson RJ, Dao ML, Lim DV. 1994. Cell-associated collagenolytic activity by group B streptococci. Infect Immun. 62: 5647-5651.








