Tutorial 1
advertisement
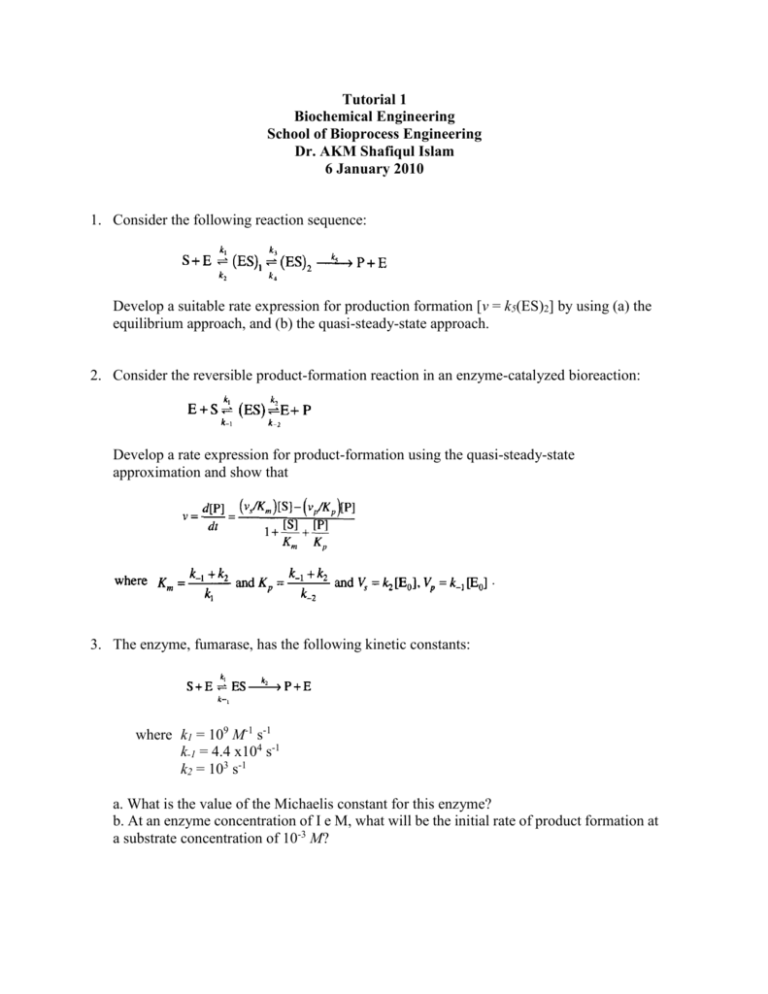
Tutorial 1 Biochemical Engineering School of Bioprocess Engineering Dr. AKM Shafiqul Islam 6 January 2010 1. Consider the following reaction sequence: Develop a suitable rate expression for production formation [v = k5(ES)2] by using (a) the equilibrium approach, and (b) the quasi-steady-state approach. 2. Consider the reversible product-formation reaction in an enzyme-catalyzed bioreaction: Develop a rate expression for product-formation using the quasi-steady-state approximation and show that 3. The enzyme, fumarase, has the following kinetic constants: where k1 = 109 M-1 s-1 k-1 = 4.4 x104 s-1 k2 = 103 s-1 a. What is the value of the Michaelis constant for this enzyme? b. At an enzyme concentration of I e M, what will be the initial rate of product formation at a substrate concentration of 10-3 M? 4. A simple mechanism of the chymotrypsin reaction is depicted below: Where E donotes chymotrypsin and S is the substrate. P1 and P2 are two products that are generated in two different steps. Suppose the substrate binding equilibrium is fast and the substrate S is in huge excess. Use steady state approximation of the intermediate (ES’) to derive the reaction rate for the generation of the products P1 and P2. 5. To learn how the rate of an enzyme-catalyzed reaction depended on the substrate concentration, a constant amount of enzyme was added to a series of reaction mixtures containing different concentrations of substrate. The initial reaction rates (velocities) were determined and the data are shown below. [Substrate] (moles / L) 5.0 x 10 -7 Velocity (moles / L - min) 0.96 5.0 x 10-6 7.1 5.0 x 10-5 20 5.0 x 10-4 25 5.0 x 10-3 25 5.0 x 10-2 25 a. What is the numerical value of Vmax for the concentration of enzyme used in these experiments? Briefly explain how you arrived at your answer. b. What is the numerical value of KM for this enzyme? Briefly explain how you arrived at your answer. c. Based on your answers to parts a and b, calculate the initial reaction rate when [Substrate] 1.0 x 10-6 mole / L. d. Suppose that the enzyme concentration in each reaction mixture were increased by a factor of 4 (compared to the concentration used to collect the data shown above). What would the numerical values of Vmax and KM be under these conditions. Briefly explain your answers.











