Ultrasensitive multiplexed biosensors using FRET in spectral and
advertisement

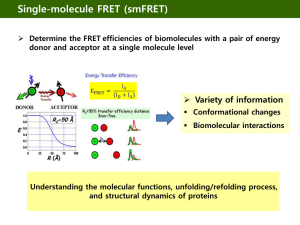
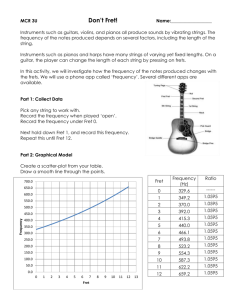
Ultrasensitive multiplexed biosensors using FRET in spectral and temporal resolution Niko Hildebrandt NanoBioPhotonics, Institut d’Electronique Fondamentale, Université Paris-Sud, Orsay, France Förster Resonance Energy Transfer (FRET) is a distance dependent non-radiative energy transfer between an excited donor and a ground state acceptor molecule or particle. As FRET occurs at distances between ca. 1 and 20 nm, it is ideally suited for the investigation of biological interactions, concentrations and/or distances. The combination of two unique FRET fluorophores, namely terbium complexes (Tbs) and semiconductor quantum dots (QDs), in time-resolved FRET offers many advantages for multiplexed biosensing [1-3]. Tbs provide long excited state lifetimes (up to several milliseconds), which allow efficient FRET to QD acceptors and nearly complete suppression of fluorescence background by time-gated detection. Spectral and temporal multiplexing becomes possible using different QD colors and the long Tb luminescence decay times, respectively. Moreover, so-called FRET relays (multistep FRET from Tb-to-QD-to-dye) allow for an even higher flexibility in the development of sophisticated biosensors. This lecture will present various Tb- and QD-based homogeneous multiplexed FRET biosensors using a variety of biological recognition molecules, such as antibodies [4,5], nanobodies [6], peptides and oligonucleotides [7,8], for multiplexed detection of tumor markers, proteases, DNA and RNA. The application of time-resolved FRET in imaging using optically multifunctional antibodies, FRET investigation of nanomedicine crossing the bloodbrain-barrier and FRET-based molecular logic devices will also be discussed. The FRET toolbox contains a myriad of different fluorophores and a large variety of steady-state and time-resolved spectroscopy and microscopy detection technologies. The efficient combination with in-vitro or in-vivo biological systems provides a highly sensitive method for multiplexed detection of biomolecular interactions. Therefore, FRET is a powerful tool for clinical prognostics and diagnostics and for a better understanding of biomolecular structures and dynamics. REFERENCES [1] B. Hötzer, I.L. Medintz and N. Hildebrandt. Small 2012, 8 (15), 2297-2326. [2] Z. Jin, N. Hildebrandt. Trends Biotechnol. 2012, 30 (7), 394-403. [3] D. Geißler, L.J. Charbonnière, R.F. Ziessel, N.G. Butlin, H.-G. Löhmannsröben and N. Hildebrandt. Angew. Chem. Int. Ed. 2010, 49(8), 1396-1401. [4] D. Geißler, S. Stufler, H.-G. Löhmannsröben and N. Hildebrandt. J. Am. Chem. Soc. 2013, 135, 1102-1109. [5] K. D. Wegner, Z. Jin, S. Lindén, T. L. Jennings, and N. Hildebrandt. ACS Nano 2013, 7 (8), 7411– 7419. [6] K. D. Wegner, S. Lindén, Z. Jin, T. L. Jennings, R. el Khoulati, P. M. P. van Bergen en Henegouwen, and N. Hildebrandt. Small 2013, DOI: 10.1002/smll.201302383. [7] W. R. Algar, D. Wegner, A. L. Huston, J. B. Blanco-Canosa, M. H. Stewart, A. Armstrong, P. E. Dawson, N. Hildebrandt and I. L. Medintz. J. Am. Chem. Soc. 2012, 134, 1876−1891. [8] W. R. Algar, A. Malonoski, K. Susumu, M. H. Stewart, N. Hildebrandt, and I. L. Medintz. Analytical Chemistry 2012, 84 (22), 10136–10146.