nmr carbon
advertisement

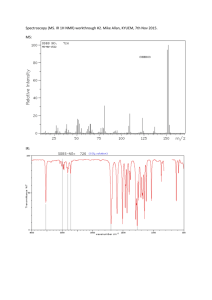
Section: 903 9/12/12 Spectroscopy- Infrared Spectroscopy and Nuclear Magnetic Resonance spectroscopy. 1. Identifying Cyclohexanone, Acetone, and Acetophenone All three compounds will have peaks around 1710 cm^-1 in their IR spectra’s. However the Cyclohexanone and Acetophenone are more substituted so they will have peaks lower than that with Acetophenone having the lowest. Acetophenone also has an aromatic ring and thus will show peaks the reflect that at 1600 cm^-1. The NMR spectra’s for each will be quite different in that the Acetone will only have one peak for its two sets of equivalent hydrogen Ions. However the Acetophenone will have a high peak around 9 ppm from its aromatic ring. These differences should clearly distinguish the compounds in a IR and NMR spectroscopy. 2. The IR spectra we did in the lab shows some clear functional groups present. The broad peak at 3300 shows clearly that this molecule has an OH alcohol group attached to it. Furthermore the peaks around 2950 suggest there are C-H3 and C-H2 stretching. This leads me to believe this was an alkyl alcohol, possibly ethanol. Section: 903 9/12/12 3. Identifying compounds a. C4H8O2 i. Molecule: Ethyl Acetate ii. Reason: It is suggested to be an alkane because the peak below 3000 cm-1 and the peak around 1710 reflects a carbonyl group. Furthermore the NMR shows a large singlet as the second most shielded and a quadruplet and triplet which suggests an ethyl group. b. C4H9Br i. Molecule: 2-Bromobutane ii. Reason: The peaks just below 3000 cm-1 suggests an alkane is present. In the NMR a weak sextet is present suggesting a single proton is adjacent to a primary carbon on one side and a secondary on the other. c. C8H9Br i. Molecule: 1-Bromo-4-ethylbenzene ii. Reason: In the IR there is a jagged peak around 3000 cm-1 that suggests an aromatic ring. The NMR has peaks between 6 and 9 ppm which further suggests an aromatic ring. d. C4H8O2 i. Molecule: Butyric Acid ii. Reason: The IR shows a broad jagged peak around 3000 cm-1 that suggests the presence of an acid. Also the peak at 1710 cm-1 further reflects the presence of a carbonyl group. The NMR shows a triplet, quadruplet, triplet sequence which suggests a propyl group. e. C5H10O i. Molecule: 3-Pentanone Section: 903 9/12/12 ii. Reason: The peak at 1710 cm-1 suggests a carbonyl group is present. The NMR shows only two peaks which suggests two sets of equal protons. f. C5H10O i. 2-Methylbutanal ii. Reason: The peak at 1710 cm-1 showed a carbonyl present. The NMR however showed 5 groups so there was no equal protons and the two groups around 1 ppm suggested a tertiary carbon next to a secondary carbon. Also the NMR peak around 10 ppm suggested a carbonyl present.