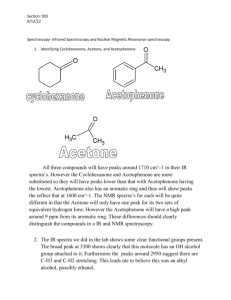
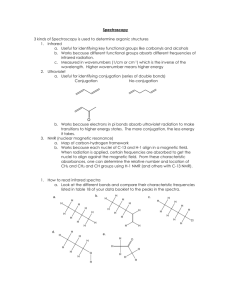
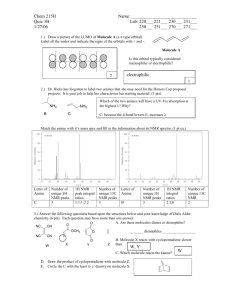
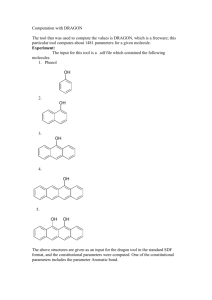
Spectroscopy workthrough2 7Nov`15
advertisement

Spectroscopy (MS. IR 1H NMR) workthrough #2. Mike Allan, KYUEM, 7th Nov 2015. MS: IR: 1H NMR: First I like to look at IR. To get a feel of the molecule. OH from alcohols, OH from carboxylic acids should be instantly recognizable (I can just 'see' the shape - not needing to look for their wavenumber). Then I look for C=O at about 1700cm-1. It's my estimation that this will be the MOST LIKELY type of compound you'll get for analysis in an exam. At this stage, bear that: just for this 1700 +/- peak alone, it could be a ketone, aldehyde, carboxylic acid, acid chloride, acyl chloride or ester. ketones, esters and anhydrides are a bit tricky to spot. The Infrared peaks of aldehydes are usually distinct and easy to spot quite quickly. two weak to medium peaks at about 2800+/- 20, and 2700. These are not on your correlation tables. If I think I have an aldehyde, or I couldn't remember the 2800 and 2700 peaks, I'll take a quick look at the 1H NMR to see if I have a peak at 9-10 ppm which will confirm of negate the presence of an aldehyde. Then I'd look for C=C (alkenes or aromatics) and if I'm not sure if I have an aromatic then I instantly look at the 1H NMR to see if I have complex multiplets at about 7.5ppb (the 1H NMR 'magic number' for aromatics') and then I will know rapidly if it's an alkene or aromatic -> I won't have to struggle to identify C=C-H 's from the IR. The weak to medium absorption of the 'double fangs' belonging to a 1o amime should also be apparent both occuring between 3300–3500 and a nice NH2 STRONG bending absorption at 1550 to 1650 cm-1 (although this latter peak isn't mentioned on your IR correlation tables). The single fang of a 2o amine is a bit more tricky to spot, occurring at about 3420 cm-1. So if I think I've spotted a 1o or 2o amine, I'll take a quick look at the MS to see if the molecular ion is an odd number. If I do see an odd number I'll be pleased and assume only 1 N containing amine group is been given (and not a higher odd number of N's e.g. 3,5,7,9... which would also give an odd number on the MS). If I see the molecular ion is a positvie number then I'll be unhappy because it may mean the milecule contains two N groups and that means the milecule will take on an extra dimension of difficulty in interpreting it, but I will try to be 'clever' and imagine that the potential 'diammine' molecule will be symmetrical - because such molecules will be much easier to interpret. If CIE gave a non-symmetrical diammine, then that would be incredibly 0mean of them, and there would be little point in them being that mean. C-N absorptions from 3o amines are found 1200 to 1350 cm-1 which is likely to be a 'busy' area of the spectrum but these peaks are probably going to be be quite strong, helping to assign one peak as a 3o amine IF NEED BE! but again these peaks are NOT on your correlation tables so you will NOT be expected to have to identify them. The extra info I'm providing here is to try and help you make progress if you are struggling by employing the simpler approach. Sometimes people just these tips and can rapidly apply them hence can come up with a structure a lot more quickly. If the extra info/tips are not useful to you, then simply ignore them. (Scroll to next page) Right... lets get cracking. TABULATE YOUR data from the table. Peak Location (cm-1) Absorption Assignment / notes A about 3440 Medium strong. This is in the same place as an alcohol but doesn't have the characteristic broadness. But we are told the sample is in CCl4 so to assign it an alcohol is fine because (having a low concentration of material) dissolved in CCl4 solvent less hydrogen bonding occurs which sharpens the peak. The important point was the location of the peak fits. B >3000 Weak v. weak peaks. looks like alkene, there are strong peaks at 1500 and 1600 also, so it's possible there's an alkene. A quick look at the NMR shows a complex multiplet between 7 and 8 so these peaks previously thought to be alkene are probably C=C-H stretches in an aromatic ring C <3000 v.weak Really lousy alkane groups (f any, but there does look like there is some CH alkane peaks). D 2800 & 2700 weak Look like CHO aldehydes peaks (although not on the CIE correlation table) E 1700 strong C=O confirming aldehyde. A quick look at the NMR gives a peak between 9 and 10 ppm, so definately an aldehyde. F Approx 1600 Strong C=C aromatic? G Approx 1500 Strong C=C aromatic? H Approx 1350 Really strong The strongest peak in the spectrum. Not immediately obvious. It doesn't fit in with any of the peaks on the CIE correlation table but the closest one is C-O. The C=C is much further away. I Approx 1140 Strong C-O possibly. At this stage I'm beginning to run flat of ideas and I don't see anything instantly helpful. The NMR conforms an aromatic and aldehyde group. The aromatic ring as a relative number of 3 in it. In the aldehyde we see raltive H count of 1 compared to the aromatics. It's doubtful there are two aldehydes which in turn would require 6 aromatic H's and that would require at least two aromatic rings or a larger single aromatic ring, so It's worth a shot to go with: One aldehyde group and 3 aromatic protons, so we are dealing with a tri substituted ring. This would give 77-2 on the mass spec. Worryingly we don't see anything significat at 75 :( but if the ring was tri substituted, then it's not beyond the realm of imagineion to suggest those three groups are making the ring fragment. One group is probably an electron withdrawing aldehyde group (assuming the aldehyde isn't on the end of a chain that's attached to the ring!) The 1H NMR tells us that a peak at about 6ppm gets smaller with addition of D2O. I'll assume this is the slightly unusual shape alcohol we assigned at about 3440cm-1 on the IR. So we think have C6H3, CHO and OH thus far. Lets tie them together in the simplest way possibile... ? O H OH C6H3, CHO and OH gives us a mass of 12x7 + 16x2 + 2 +3 = 121 Now, mass spec: I've already got 8 C's from my proposed molecule already hence I'll have an (M+1)+ peak from 13C isotopes. I think that (M+1)+ peak is at 153. I'll take 152 as the molecular mass then. There is a slightly smaller peak at 151 to the left, so that's going to be the molecule minus a H atom. Maybe from the aldehyde or the alcohol group (note: aromatic alcohols have their molecular ion surviving more than aliphatic ones). I can see lots of peak clusters, so this is variations of various fragments. Clustering is quite common with fragmented aromatic rings. As molecular ion = 152, I am missing a mass of 152 - 121 from what I think is a third substituent on the ring. i.e. I'm 'missing' a mass of 31 units. That'll be 2 carbons maximum, but then I'll have 11 H's left over and that's too much. Instead of having two C's in that mass 31, then maybe I have an oxygen. A quick look at the IR does allow me to think there is a C-O. C-O will take up 28 mass units leaving me with 3 units of mass, which can easily be stuck on the atoms. I might have CH2-OH coming from the ring or CH3-O (a 'methoxy' group) and I have no info of TWO peaks diminishing on the 1H NMR, so I'll go with the CH3-O coming from the ring for now. This gives me a working molecule to test... H3C O O H OH Peak Location (cm-1) Assignment / notes p 153 (M+1)+ Q 152 M+ R 151 (M-H)+ R 151 (M-H)+ 137 Loss of 15 mass units from M+. This could be the molecule minus a CH3 group. 123 Loss of a mass of 30 from M+. This could be the CHO breaking off along with loss of an additional H broken off from the OH 114 Loss of 38 from the molecule, or loss of some lesser mass from other fragments. Not easy to assign this one in terms of the 'side' groups coming off the ring, so it's could be a fragment containing a part of the fragmented ring with one or two of the other groups on it. Say M+ - CH=COH = 152 24+16+2 = 152 - 42. So close. Maybe the fragment is C=C-O = 24+16 = 40. No so maybe another fragment... This one isn't easy to assign so I'll skip over it for now... 81 This is M+ - 71 mass units. It's fair to say the molecules has split almost exactly in half. to get mass 81 with remainder 71. 81=36+12+16+3+17=84 = too heavy... 24+1+32+12+2 = 71 O-C-C-C-CHO = 1+16+12+12+!2+12+1+16 = 71 so maybe 81 is the remaining atoms, but it's not easy to come up with a fragment so easily. 65 51 39 27 15 v. small but suggests CH3 Assigning plausible M.S. fragments from the proposed structure is proving difficult, so I'll jump back to the NMR. Peak Location (ppm) Relative Integration height Coupling / splitting pattern Assignment Ha about 9.8 1 singlet An aldehyde. Hb about 7.42 1 Cannot easily determine splitting Two aromatic H's are stated to be in here. They must have the same environment as they have the same value. Hc about 7.42 1 Cannot easily determine splitting Same as above. Hd about 7.05 1 singlet An single an aromatic H in a different environment from the other two (see above). Seems to be split into 2 therefore next to one other H - one of the H's above. He about 6.39 1 singlet Alcohol (peak diminishes on D2O and little or no evidence of COOH or amine on other spectra) Hf about 4 1 singlet CH3 on a O -O-CH3 (aromatic methoxy) Perhaps then we could have variations on the ring pattern. e.g. H3C O O H OH H3C H3C O O O H3C O H H O O HO H HO OH O O H3C O O H H OH H3C O OH H OH H3C O H3C O O H OH O H H3C O O H3C O H HO OH Which of these structures is closest to the aromatic H's pattern? Hint... some of the information in the complex aromatic region may be overlapping... Maybe you can't deduce the exact ring substitution, but in an A-level exam you would probably get max marks IF you proposed any one of them because this exercise was very hard with four functional groups. Admittedly, rings are pretty tricky to get correct. In a CIE exam you'll probably be given a much more friendly molecule - probably aliphatic, but if you did get a ring it will probably be more simple than the above. It will probably me monosubstituted, 1,4-disubstituted (hence symmetrical!) or some other combination of symmetry 1,3,5-trisubstituted. The actual molecule is the very last one drawn. We couldn't get good fragments because we didn't have the right substitution on the ring. Go back now and try and assign fragments.