Spectroscopy Review Problems
advertisement

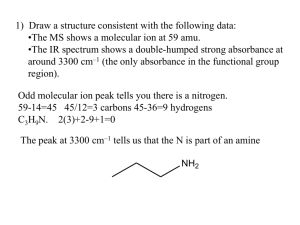
SPECTROSCOPY PROBLEMS Dr. Sheppard CHEM 2412 Fall 2014 McMurry (8th ed.) sections 12.1-3, 12.5-8, 13.1-5, 13.7-11, 13.13 1. Which of the following statements best describes the base peak in a mass spectrum? A. The peak from the most stable radical. B. The peak from the species that has the isotope with the highest atomic number. C. The peak of highest intensity. D. The peak from the molecule minus an electron. 2. This compound contains C, H, and one other atom. Identify the other atom from the mass spectrum and explain your reasoning. 3. Consider the mass spectrum of 2methylbutane. (a) What peak represents M+? (b) What peak represents the base peak? 4. True or false, both mass spectrometry and infrared spectroscopy involve the interaction of molecules with electromagnetic energy? 5. The amount of energy in infrared light corresponds to: A. the amount of energy needed to promote one electron from a bonding to an antibonding molecular orbital B. the amount of energy needed to "flip" the spin of a 13C or 1H nucleus C. the amount of energy needed to strip a molecule of one electron to generate a cation radical D. the amount of energy needed to increase certain molecular motions, such as bond vibrations, in organic molecules 6. Examining the infrared spectrum of a compound allows us to: A. determine the types of functional groups present in the compound B. determine the carbon-hydrogen framework of the compound C. determine the molecular weight of the compound D. determine the nature of the conjugated pi electron system in the compound 7. Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur. Place the letter of the region in the blank to the left of the bond-type. _____ C-C, C-O, C-N, and C-X single-bond vibrations. _____ C=O, C=N, and C=C bond absorptions. _____ N-H, C-H, and O-H stretching and bending motions. _____ triple bond stretching vibrations. A. 4000 to 2500 cm-1 B. 2500 to 2000 cm-1 C. 2000 to 1500 cm-1 D. below 1500 cm-1 8. When 1-bromopropane reacts with ethoxide ion, two products are formed. Write the structures of both products, and tell how they could be distinguished using IR spectroscopy. 9. Assume you are carrying out the conversion of 1-bromobutane to 1-butanol. How could you use IR spectroscopy to determine when the reaction is complete? 10. Match a structure from the list below to the following IR spectra. 11. Match a structure from the list below to the following IR spectra. 12. Match a structure from the list below to the following IR spectra. 13. Name the type of spectroscopy shown in each spectrum. A C B D 14. Match a term to each description below. When looking at an NMR chart the right-hand part of the chart is the _____. The exact place on the chart at which a nucleus absorbs is called its _____. The calibration standard for 1H and 13C NMR is _____. The NMR charts are calibrated using an arbitrary scale that is divided into _____ units. A. B. C. D. E. F. G. TMS high-field or upfield side MHz delta (d) low-field or downfield side chemical shift specific absorption 15. For each of the compounds below tell how many signals you would expect the molecule to have in its normal, H-decoupled 13C NMR spectra. 16. For each compound below tell how many types of nonequivalent protons there are. 17. Predict the splitting patterns you would expect for each proton in the molecules below. 18. What is the splitting pattern for the hydrogens in 3-methyl-2-butanone labeled a? A. septet B. quartet C. doublet D. singlet 19. What is the splitting pattern for the hydrogens in 3-methyl-2-butanone labeled b? A. septet B. quartet C. doublet D. singlet 20. Treatment of tert-butyl alcohol with hydrogen chloride yields a mixture of tertbutyl chloride (SN1 product) and 2methylpropene (E1 product). After chromatographic separation, how would you use 1H-NMR to help you decide which was which? 21. Propose a structure that fits the following 1H-NMR data. • C8H9Br • 3H doublet at d2.0 • 1H quartet at d5.0 • 5H multiplet at d7.3 22. Propose a structure for this compound. • C4H8O 23. Which structure of molecular formula C4H8Cl2 fits both the 1H-NMR and 13C-NMR spectra shown below? A B C D 24. Calculate the Index of Hydrogen Deficiency (degree of unsaturation) for a molecule with the molecular formula C5H12O.