Exam 2 Topics and Study Tips/Strategies
advertisement

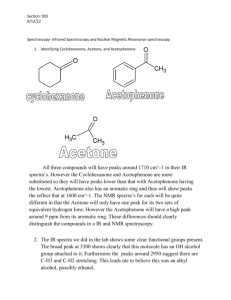
Exam 2 Topics and Study Tips/Strategies Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Hannah J. Chem 331 Dr. Winter 10/4/15 Exam 2 Topics: The following is a (checkable) list of the topics you should know and understand for exam 2: Ch. 5: What a plane of symmetry is and how to find it/them in a molecule Which molecular geometries do and don’t superimpose on their mirror images Definition and identification (i.e. from R/S notation) of enantiomers. Given a molecule, you should be able to draw an enantiomer. Also, know which physical property differs between enantiomers (and that it is the only one that is different). Definition and identification of a chiral center. Know R & S naming: o Purpose/for which kinds of molecules it is used o How to assign R/S naming either by rotating the molecule on paper or using the thumb rule (pick whichever you can use efficiently and correctly) o Be able to name whole molecules, including R & S notation Definition of a racemic mixture or racemate Definition and identification (i.e. from R/S notation) of diastereomers. Given a molecule, you should be able to draw a diastereomer. Know that only molecules with multiple chiral centers can be diastereomers. Also, know which physical property/properties differ between diastereomers. Definition of a meso compound Know the formula for the maximum number of stereoisomers in a molecule and be able to determine the actual number of unique stereoisomers present in a molecule based on symmetry. Ch. 12: Mass Spectrometry o Have a simple conceptual understanding of how mass spectrometry works and what we use it to find (the exact (to about 4 decimal places) molecular weight of the molecule, which enables us the find the formula). Also know what and where on the spectrum the M+, or molecular ion, peak is and what we use it for (see above parentheses). o Know why we consider the radicals that a mass spectrometer registers to have the same molecular weight as their uncharged counterparts. o Be able to explain why there is a small peak 1 g/mol greater than the M+ peak (based on 13 C isotopes). Infrared (IR) Spectrometry o Have a simple conceptual understanding of how infrared (IR) spectrometry works and what we use it to find (functional groups). o Know what and where the fingerprint region is and that we always ignore it. o Know the peak shapes associated with a carbonyl, alcohol, and amine (primary, secondary, etc.). Especially know that alcohol is a big, broad peak around 3500 cm-1. o Be comfortable using an IR chart (like the one on page 439 in the textbook) to identify functional groups from an IR spectrum or written peak locations. Be able to distinguish Mass, IR, and NMR spectra when you’re not told which is which Ch. 13: 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu Have a simple conceptual understanding of how Nuclear Magnetic Resonance (NMR) spectrometry works and what we use it to find (the structure of a molecule) Understand the difference between 1H and 13C NMR and know that we primarily use 1H (know why) Know which end is downfield and upfield, high and low chemical shifts, high and low frequency, more and less shielded and why a peak registers more or less downfield based on the electronegativity of the atom it’s connected to Understand that we measure NMR spectra relative to a standard (TMS) to even out results for more and less expensive NMR spectrometers (better and worse magnets used) and that that’s why frequency is given in ppm (parts per million). Be able to identify how many peaks a given molecule will have in either an 1H or 13C NMR spectrum based on total number of H’s/C’s and symmetry. Also, know what it means for pieces of a molecule to be chemically equivalent and how to determine chemical equivalency Understand and be able to determine relative integration for the different peaks of a molecule Coupling: what it is, which H’s do and do not couple with each other, and be able to determine the number of peaks/coupling pattern (singlet, doublet, etc.) for any H in the molecule using the n + 1 rule. Also, know that OH and NH do not couple (they are always singlets) and are not included in coupling counts for neighboring H’s Understand the distinction between relative integration and coupling (so you don’t get them confused). Be comfortable using an NMR chart (like the one on page 475 in the textbook) to identify what each peak from an NMR spectrum or written peak locations represents, including the aromatic ring (Ar or Aryl) and halide (Hal = F, Cl, Br, I) abbreviations. Be comfortable determining structure from either the NMR, IR spectra or just the written peak locations (with the δ notation—also understand how relative integration (e.g. 2H, 6H, etc.) and coupling (e.g. s, d, etc.) are given in the written version) Know how to determine degrees of unsaturation (DOU) and what the number means. Know also that a DOU of 4 or greater almost always means that there is an aromatic ring in the molecule. KEY: Be Able to Determine Structures from 1H NMR (possibly also given IR) Spectra. Use the “detective/clue” 5-step method given in class or another that works reliably. Do plenty of practice problems on this! Study Tips/Strategies: Go through your notes, reading them and highlighting important topics and details (This is a good first step in studying for any exam. If you don’t have good notes, ask a friend.) Redo in-class examples and/or SI worksheets without looking at the solutions, then check your work Do end-of-chapter problems (Ch. 5, 12, 13) in textbook and/or google simple IR/NMR problems Read end-of-chapter summaries and passages on confusing topics in textbook Form a study group and study together Make flashcards for the memorized items and go through them until you can identify the opposite side both ways in any order on the first try Finish all applicable Sapling homework & look back through past assignments Come to SI DO THE PRACTICE EXAM POSTED ON BLACKBOARD (if it’s helpful to you, consider taking it once without studying to gauge where you are, then retake it after studying) Make a list of reminders about things you might forget (e.g. Label formal charges, calculate D.O.U. first on NMR problems) Ask a friend, me (your SI leader), or Dr. Winter in person or via email about any topics that you are still confused on after studying Power through and don’t forget to breathe! If you’ve been putting in the effort and gotten a good grasp on the material, you’ll be successful on the exam—you can do it!